CH 1012 LECTURE 2 LAST LECTURE What is



- Slides: 15

CH 1012 LECTURE 2 LAST LECTURE • What is involved in the course • Inorganic chemistry : main group, Tmetal • Periodic table – different groups • Materials: elements, compounds, • Phases: gas, liquid, solid • Ionic solids: metal+non-metal, electrostatic attraction, Na. Cl , 3 D-lattice of ions • Molecules: discrete chemical units • Covalent bonding : non-metal + non-metal, sharing e- pair, increased e- r, distance fixed • Chemical equilibrium : K; Le Chatelier’s Principle: net rxn opposes change. L 1 / 1

CH 1012 COLLOIDS Xe atoms 5 nm tall Dispersed System • any homogeneous medium >0. 3 mm (dispersion medium) containing dispersed particles (disperse phase). HEPA Filter Ostwald (1900): World of Neglected Dimensions Colloids Coarse Disp. Colloid Molecular Disp. > 1 mm - 10 nm < 10 nm std. filters ultrafilters not retained optical electron scanning tunneling microscope micro. 2

CH 1012 WHY DISPERSED SYSTEMS? Why dispersed systems are of interest Many biological structures are colloidal eg. blood red blood cells in serum Many mineral types are complex sols eg. oil bearing rock finely dispersed oil/rock New Technology relies on the unique properties of colloids. eg. Cotrell precipitator Aerosols 4

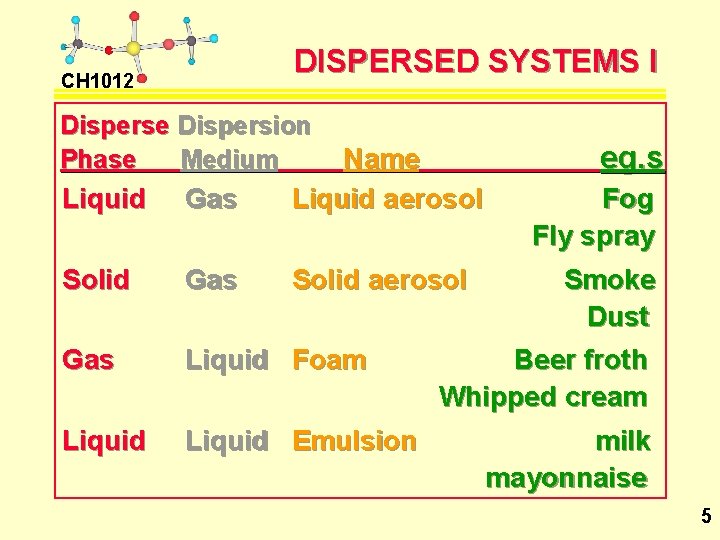
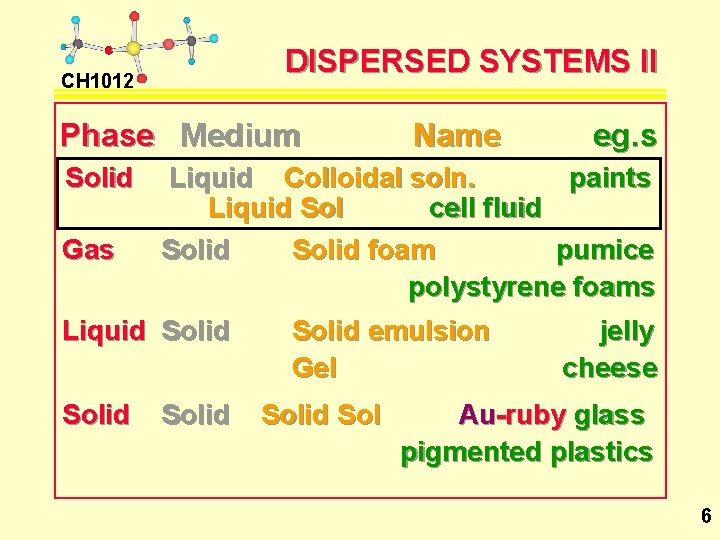
DISPERSED SYSTEMS I CH 1012 Disperse Dispersion Phase Medium Liquid Gas Name Liquid aerosol Solid Gas Solid aerosol Gas Liquid Foam Liquid Emulsion eg. s Fog Fly spray Smoke Dust Beer froth Whipped cream milk mayonnaise 5

DISPERSED SYSTEMS II CH 1012 Phase Medium Solid Gas eg. s Liquid Colloidal soln. paints Liquid Sol cell fluid Solid foam pumice polystyrene foams Liquid Solid Name Solid emulsion Gel Solid Sol jelly cheese Au-ruby glass pigmented plastics 6

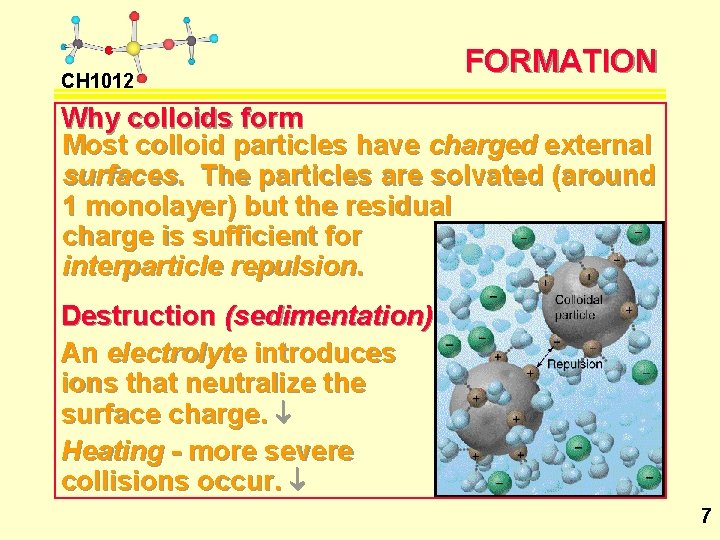
CH 1012 FORMATION Why colloids form Most colloid particles have charged external surfaces. The particles are solvated (around 1 monolayer) but the residual charge is sufficient for interparticle repulsion. Destruction (sedimentation) An electrolyte introduces ions that neutralize the surface charge. ¯ Heating - more severe collisions occur. ¯ 7

CH 1012 BROWNIAN MOTION Brownian Motion Viewed under low magnification colloidal particles change speed and direction erratically in a characteristic way that is referred to as Brownian motion. Brown used pollen in H 2 O • Collisions of the colloid particles with the dispersing medium causes this motion. • A. Einstein explained Brownian motion using a molecular model in 1905. • These collisions provide the energy for aggregation and collapse of the colloid. A process called flocculation. 423 o. C liquid Pb in solid Al ! 8

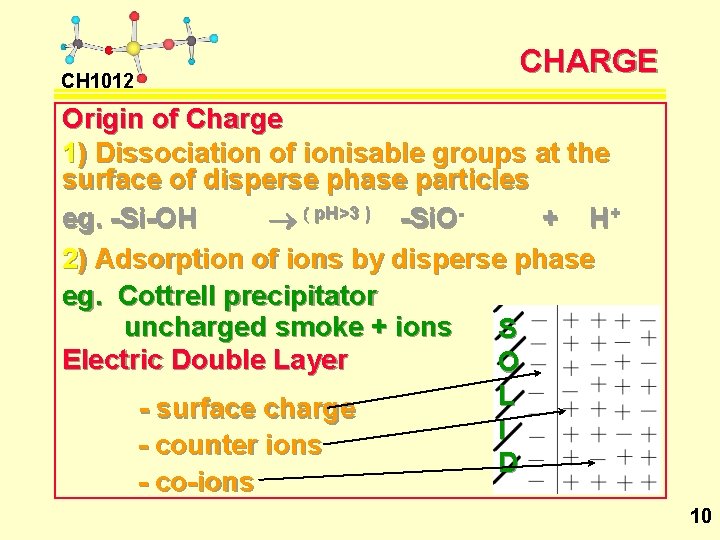
CH 1012 CHARGE Origin of Charge 1) Dissociation of ionisable groups at the surface of disperse phase particles eg. -Si-OH ® ( p. H>3 ) -Si. O+ H+ 2) Adsorption of ions by disperse phase eg. Cottrell precipitator uncharged smoke + ions S Electric Double Layer O L - surface charge I - counter ions D - co-ions 10

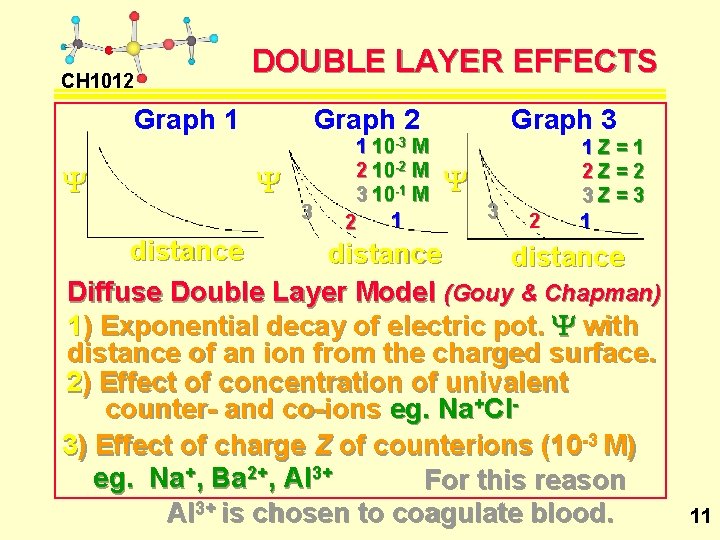
CH 1012 DOUBLE LAYER EFFECTS Graph 1 Y Graph 2 Y 3 1 10 -3 M 2 10 -2 M 3 10 -1 M 1 2 Graph 3 Y 3 2 1 Z=1 2 Z=2 3 Z=3 1 distance Diffuse Double Layer Model (Gouy & Chapman) 1) Exponential decay of electric pot. Y with distance of an ion from the charged surface. 2) Effect of concentration of univalent counter- and co-ions eg. Na+Cl 3) Effect of charge Z of counterions (10 -3 M) eg. Na+, Ba 2+, Al 3+ For this reason Al 3+ is chosen to coagulate blood. 11

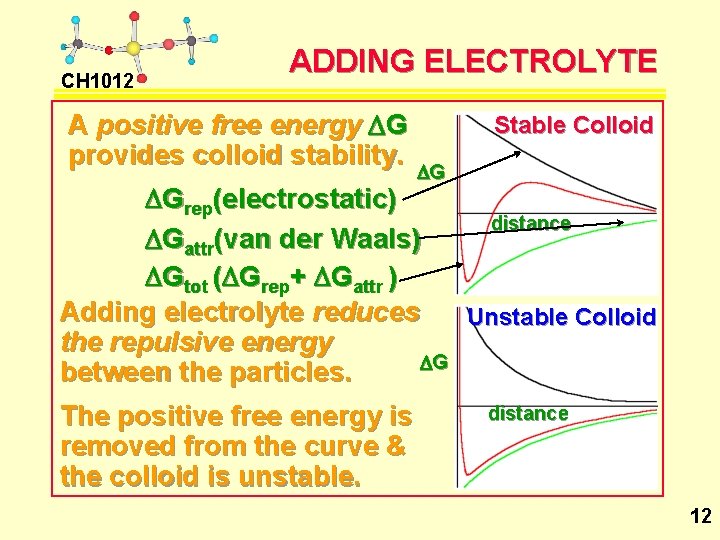
CH 1012 ADDING ELECTROLYTE A positive free energy DG provides colloid stability. Stable Colloid DG DGrep(electrostatic) distance DGattr(van der Waals) DGtot (DGrep+ DGattr ) Adding electrolyte reduces Unstable Colloid the repulsive energy DG between the particles. The positive free energy is removed from the curve & the colloid is unstable. distance 12

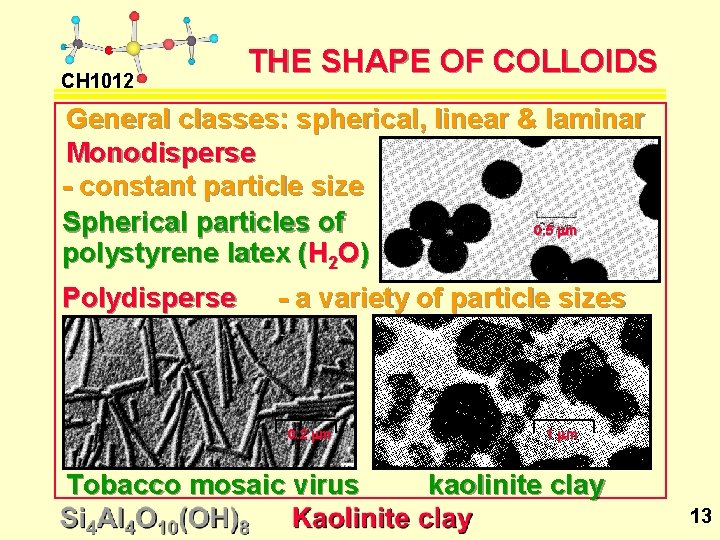
CH 1012 THE SHAPE OF COLLOIDS General classes: spherical, linear & laminar Monodisperse - constant particle size Spherical particles of 0. 5 mm polystyrene latex (H 2 O) Polydisperse - a variety of particle sizes 0. 2 mm Tobacco mosaic virus 1 mm kaolinite clay 13

CH 1012 SURFACE AREA Surface Area Available surface area determines reactivity and adsorption properties for a particle. The ratio: S. A. / Volume is high for colloids. Length of side Number of Total Surface of cubes area of cubes 1 cm 1 6 cm 2 1 mm 103 (in 1 cm 3) 60 cm 2 Colloids 1 mm 1012 6 m 2 10 nm 1018 600 m 2 14

CH 1012 SOME EXAMPLES TO TRY 1) What are two way that we can distinguish colloid particles from particles in true solution? 2) Use the Gouy-Chapman model of the diffuse double layer to predict why the addition of 1. 0 M Ba. Cl 2 to a colloid is more successful than the addition of a 1. 0 M solution of Na. Cl in causing the colloid to flocculate. L 1 / 15

CH 1012 WORKING I 1) What are two way that we can distinguish colloid particles from particles in true solution? • Colloid particles can be trapped on an ultrafilter whereas particles in true solution can’t. The colloid particles can be seen using electron microscopy whereas those in true solution are too small and remain invisible. L 1 / 16

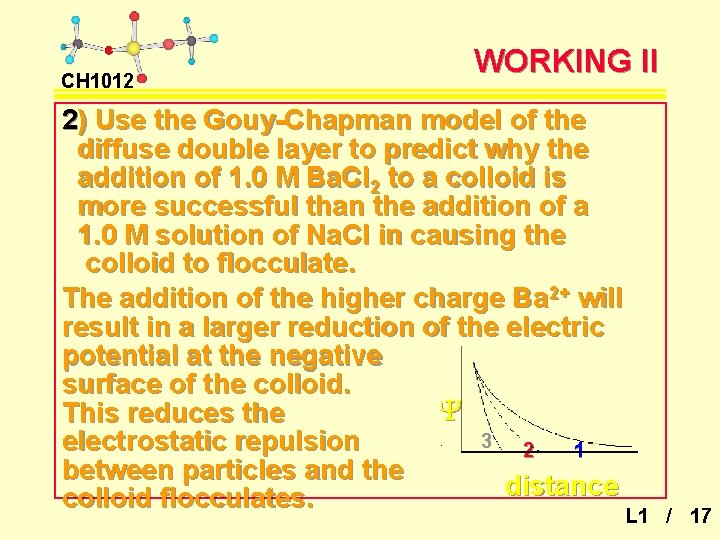
CH 1012 WORKING II 2) Use the Gouy-Chapman model of the diffuse double layer to predict why the addition of 1. 0 M Ba. Cl 2 to a colloid is more successful than the addition of a 1. 0 M solution of Na. Cl in causing the colloid to flocculate. The addition of the higher charge Ba 2+ will result in a larger reduction of the electric potential at the negative surface of the colloid. Y This reduces the 3 electrostatic repulsion 2 1 between particles and the distance colloid flocculates. L 1 / 17