August 8 2019 Kristel Schorr Pavan Agarwal Jihwang































- Slides: 44

August 8, 2019 Kristel Schorr Pavan Agarwal Jihwang Yeo Digital Health: Overview of Technology, Business, and Legal Trends and Issues

What is Digital Health? § Partnership between technology and healthcare § Use of technology to gather, exchange and manage data for healthcare services, including prevention/monitoring, diagnosis and treatment/intervention. – Wearable devices (e. g. , Apple Watch, Fit. Bit) – data provided to user and can be shared with provider – Mobile apps (e. g. , Health. Kit platform, Health app, Apple’s Health Records, Medtronic’s pacemaker app) – expanding the range of what users can monitor, transition from “lifestyle” to medical as more sensors develop. – Digital diagnostics and therapeutics (e. g. , “smart” bandages to detect wound healing and infection, deliver drugs) – Ingestible sensors – pill sized electronics that provide data to smartphone after ingestion – Augmented and virtual reality (e. g. , Microsoft’s Holo. Lens) – 5 G

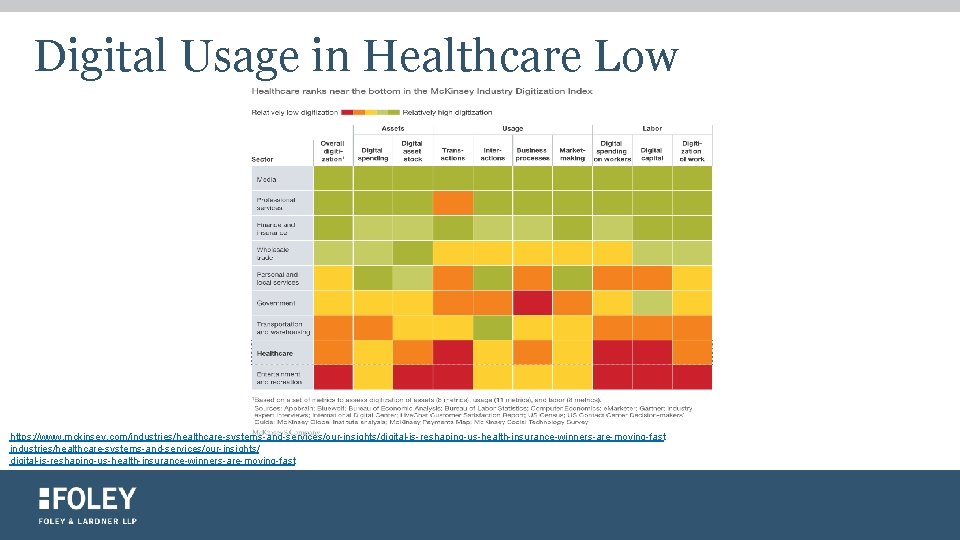
Digital Usage in Healthcare Low https: //www. mckinsey. com/industries/healthcare-systems-and-services/our-insights/digital-is-reshaping-us-health-insurance-winners-are-moving-fast industries/healthcare-systems-and-services/our-insights/ digital-is-reshaping-us-health-insurance-winners-are-moving-fast

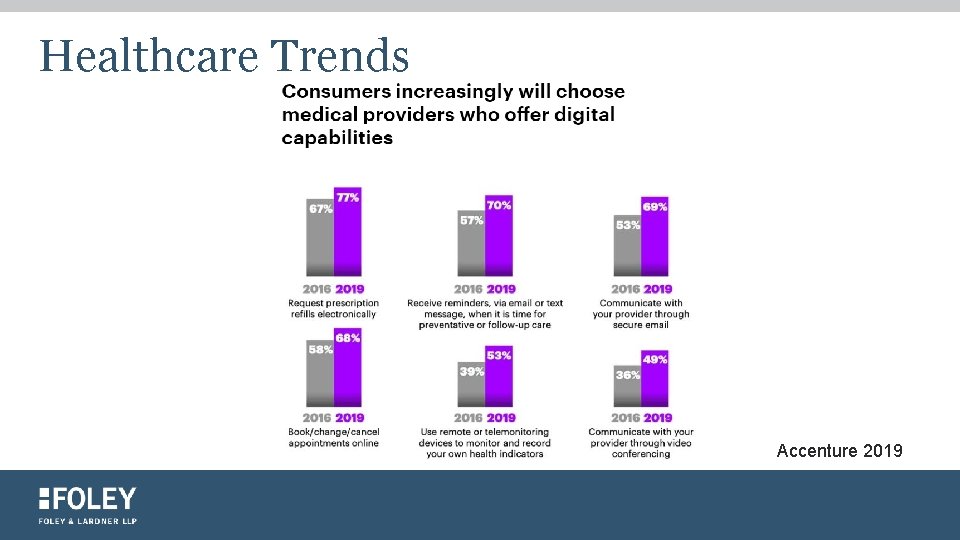
Healthcare Trends Accenture 2019

Trends (cont’d) § § § Physician-patient interaction – Clinicians extend care beyond their office – Improve patient outcomes through higher level of patient engagement (e. g. , patient can track health and wellness related activities). Relationship between insurers and patients – Insurer cost can be cut by fewer physician appointments, emergency room visits, hospital admissions, which savings can lower premiums – Insurer denial of reimbursement? Big data analytics – Medical/clinical databases – AI

Trends (cont’d) § Health delivery system – Telemedicine – delivery of health service when patient health care worker separated by a distance (patient to provider) or provider to provider § younger patients looking for more digitally savvy care, decreased cost and convenience – Reducing administrative burden on physicians by remote patient monitoring

Overall Impact § Drive healthcare costs down – Patient engagement fewer physician visits § Reduce risk of disease with behavior – Amass data to identify links between disease and lifestyle with AI § Earlier diagnosis and early treatment § Develop better medicines § Develop patient tailored medicines/precision medicine – Reduce inefficiencies

Legal and Business Considerations § Patient/data privacy and consent – selling data from wearables – HIPAA protects the privacy and security of certain health information and requires certain entities to provide notifications of health information breaches. § Data security – healthcare breach concerns § Regulatory – FDA and FTC – FDA regulates the safety and effectiveness of medical devices, including certain mobile medical apps - focuses on a small subset of health apps that pose a higher risk if they don’t work as intended. – FTC requires, inter alia, certain businesses to provide notifications following breaches of personal health record information.

Legal Considerations (cont’d) § Licensing and M&A/technology transactions – § Intellectual property – § Power supply (battery development), software and hardware development (apps, devices), etc. Healthcare – § Collaboration - finding right partner Medicare/Medicaid insurance coverage if physician intervention but no clinic visit? Product liability – Defective hardware or software, or incorrect input data, may result in harm being caused to patients/users

US – FDA’s Digital Health Action Plan § July 2017 regulation plays critical role in innovating digital health products – FDA does not want to inhibit development of technology – Ensure patients have “timely access to high quality, safe and effective digital health products” § Goals – Increasing the number and expertise of digital health staff at FDA – Launching digital health software precertification pilot program (“Pre-Cert”) – Issuing guidance to modernize policies.

Pre-Cert § Sept 2017, nine diverse companies have been selected to participate – After reviewing systems for software design, validation and maintenance, FDA can determine whether the company meets quality standards and if so, to pre-certify the company. – Program is intended to identify key metrics and performance indicators needed for a software developer or digital health technology developer to be pre-certified and ultimately potentially submit less information to the FDA than is currently required under the formal program. § Small startups and large companies, high- and low-risk medical device software products, medical product manufacturers and software developers.

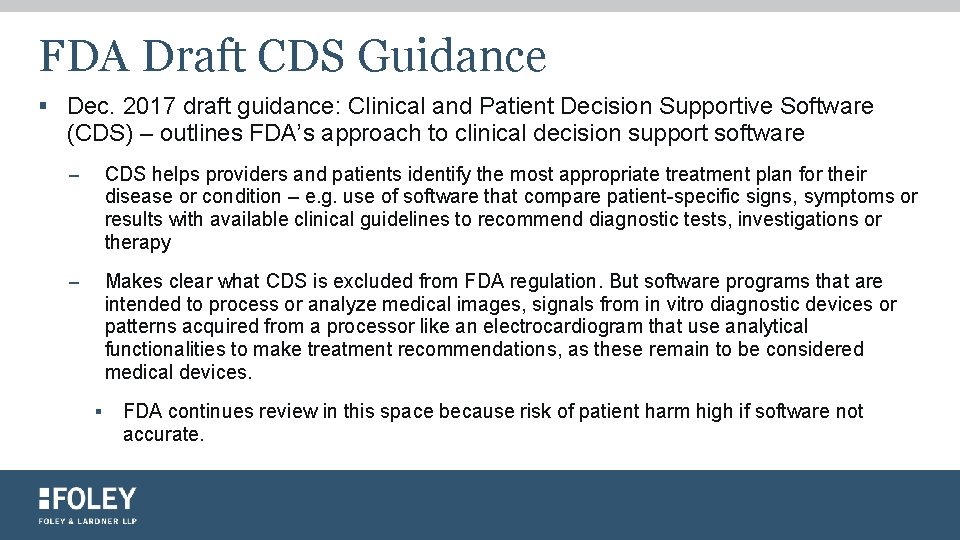
FDA Draft CDS Guidance § Dec. 2017 draft guidance: Clinical and Patient Decision Supportive Software (CDS) – outlines FDA’s approach to clinical decision support software – CDS helps providers and patients identify the most appropriate treatment plan for their disease or condition – e. g. use of software that compare patient-specific signs, symptoms or results with available clinical guidelines to recommend diagnostic tests, investigations or therapy – Makes clear what CDS is excluded from FDA regulation. But software programs that are intended to process or analyze medical images, signals from in vitro diagnostic devices or patterns acquired from a processor like an electrocardiogram that use analytical functionalities to make treatment recommendations, as these remain to be considered medical devices. § FDA continues review in this space because risk of patient harm high if software not accurate.

FDA Draft Medical Software Guidance § Dec. 2017 draft guidance: Changes to Existing Medical Software Policies Resulting from Section 3060 of the 21 st Century Cures Act § Outlines the FDA’s interpretation of the types of software that are no longer considered medical devices. – Certain digital health technologies (e. g. , mobile apps intended only for maintaining or encouraging a healthy lifestyle generally fall outside the scope of FDA’s regulation) - pose a low risk to patients but provide great value to consumers and the healthcare system.

FDA Final Guidance – Sa. MD § Dec. 2017 final guidance: Software as a Medical Device (Sa. MD): Clinical Evaluation – Establishes common principles for regulators to use in evaluating the safety, effectiveness and performance of Sa. MD. – Provides globally recognized principles for analyzing and assessing Sa. MD, based on the overall risk of the product. The agency’s adoption of these principles provides us with an initial framework when further developing our own specific regulatory approaches and expectations for regulatory oversight, and is another important piece in our overarching policy framework for digital health.

Federal Trade Commission § FTC has tool to help developer of mobile app that collects, creates or shares information to discern which federal laws apply to satisfy compliance obligations. – https: //www. ftc. gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool

EU – Medical Devices Regulation § MDR – update to Medical Device Directive (MDD), governs the production and distribution of medical devices in Europe – Final document published in May 2017 but not into effect until May 2020 § MDD provided for medical device companies to obtain CE marking and get to market. The MDR encourage policies and procedures that heighten the responsibilities of medical device companies for their products throughout the product lifecycle. § Compliance requirements for certain medical devices.

WHO – Digital Health Guidelines § WHO releases first digital health guidelines April 2019 Digital interventions for health system strengthening – § Outlines 10 recommendations on how to use digital health tools to support patients' health outcomes and access to care. § Specific to certain contexts/conditions Select recommended interventions to be accessible at least by mobile app: – § Digital birth and death notifications § Client to provider and provider to provider telemedicine § Digital client communications § Digital tracking of client’s health status

Examples of Industry Collaborations § Pharma and software start ups – Propeller Health and Boehringer Ingelheim – GSK and Propeller Health – produce sensors to collect and record data re usage for GSK dry powder inhaler – Otsuka and Proteus Digital Health - sensor embedded in Otsuka’s drug for schizophrenia, bipolar and major depressive disorder works in tandem with a patch worn on the skin (drug-device combination) § Qualcomm and Novartis - Novartis is working with Qualcomm Life’s 2 net Platform, 2 net Hub and 2 net Mobile technologies with certain medical devices to automate data from patient’s homes during clinical trials. § Apple and Zimmer Biomet -creates medical products to track how people recover from surgery § United. Health Group acquired Patients. Like. Me = online patient community portal ffers those with a chronic condition the opportunity to track and share symptoms and treatment experiences, contribute data for research and connect with others who are going through similar situations.

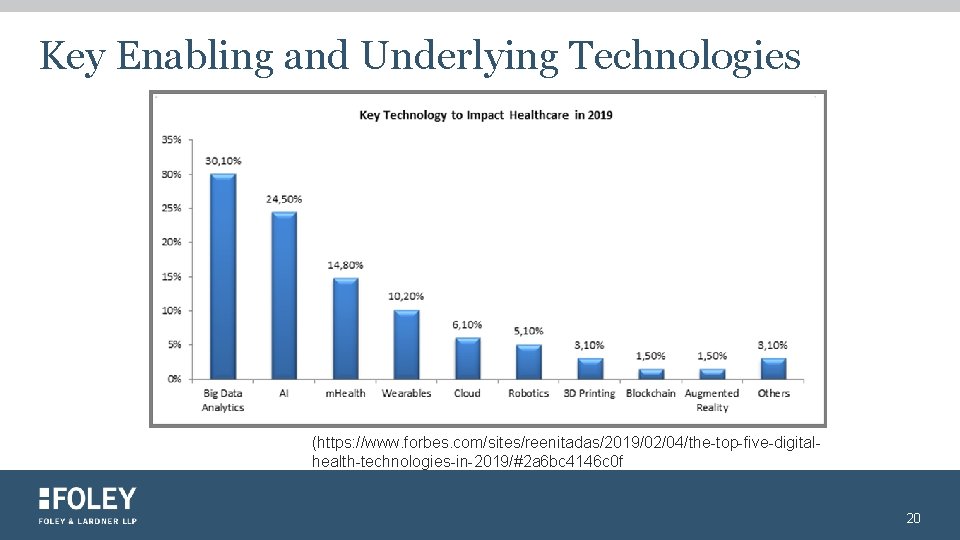
Key Enabling and Underlying Technologies § Big Data § m. Health and wearables § Artificial Intelligence (AI) § Virtual Reality (V/R) § Blockchain 19

Key Enabling and Underlying Technologies (https: //www. forbes. com/sites/reenitadas/2019/02/04/the-top-five-digitalhealth-technologies-in-2019/#2 a 6 bc 4146 c 0 f 20

Key Technologies – Big Data § Digital Health Big Data: Use of data science techniques to capture and analyze huge and complex datasets in order to positively impact patient care outcomes, and optimize business processes. https: //innovatemedtec. com/digital-health/big-data § Examples of big data: – Biometric data collected from users, such as from wearables – Medical record data – electronic medical records, other healthcare records, claims and billing records – Pharma data – data on drugs, clinical trials, patient interactions Companies are seeking new ways to leverage the vast amounts of data being collected to provide more efficient and personal patient solutions 21

Key Technologies - Wearables https: //mobisoftinfotech. com/resources/blog/wea rable-technology-in-healthcare/ 22

Wearables - Market Growth Reports and Data: The global wearable healthcare/medical devices market is expected to reach USD 27. 49 billion by 2026 https: //www. globenewswire. com/newsrelease/2019/02/27/1743565/0/en/Wearable-Healthcare-Medical-Devices-Market. To-Reach-USD-27 -49 -Billion-By-2026 -CAGR-18 -5 -Reports-And-Data. html IDC: “Wearables stand to play an important role in digital health, constantly collecting important patient data while also giving patients the ability to selfmonitor. ” https: //www. wearable-technologies. com/2019/03/idcforecasts-double-digit-growth-in-wearables-withsmartwatches-and-wireless-headphones-leading-the-way/ 23

Key Technologies - m. Health Apps My Dose Coach – For adults with Type 2 diabetes – tracks fasting blood glucose level. Provides dose recommendations to adjust insulin dose. Go. Spiro App - Pairs spirometer with mobile app. Spirometer measures air output from the lungs. App connects wirelessly to device to collect full spirometry data and provide lab quality results for in-home clinical trials. (Virtual Clinical Trials) re. SET - For Substance Use Disorder (SUD) intended to provide cognitive behavioral therapy. (Prescriptive Digital Therapeutic) 24

m. Health Apps – Sa. MD Software as a Medical Device (Sa. MD) - "software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device. " International Medical Device Regulators Forum (IMDRF) Examples: § Software that enables clinical communication and workflow § Software used to "drive or control" the motors and the pumping of medication § Software that monitors performance or proper functioning of a device 25

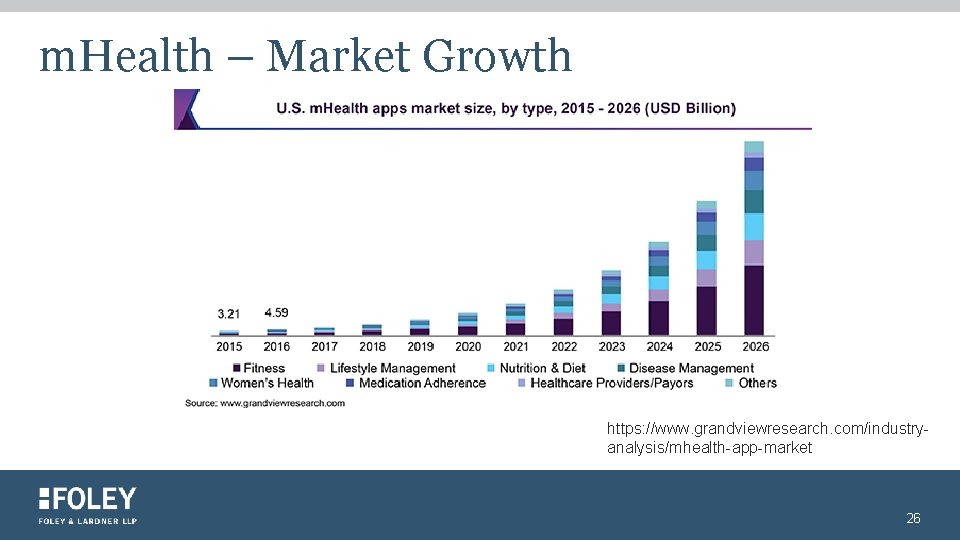
m. Health – Market Growth https: //www. grandviewresearch. com/industryanalysis/mhealth-app-market 26

Key Technologies – AI (Artificial Intelligence) • • • Deep Neural Network (DNN) Convolutional Neural Network (CNN) Deep Learning • • Artificial neural networks Decision trees Support vector machines Bayesian networks Genetic algorithms • • • Reasoning Knowledge representation Planning Learning Natural language processing Perception Machine Learning AI 27

Key Technologies – ML (Machine Learning) Neuron Train NN ANN (Artificial Neural Network) No Evaluate – Satisfied? Yes DNN (Deep Neural Network) Execute Trained NN → ? 28

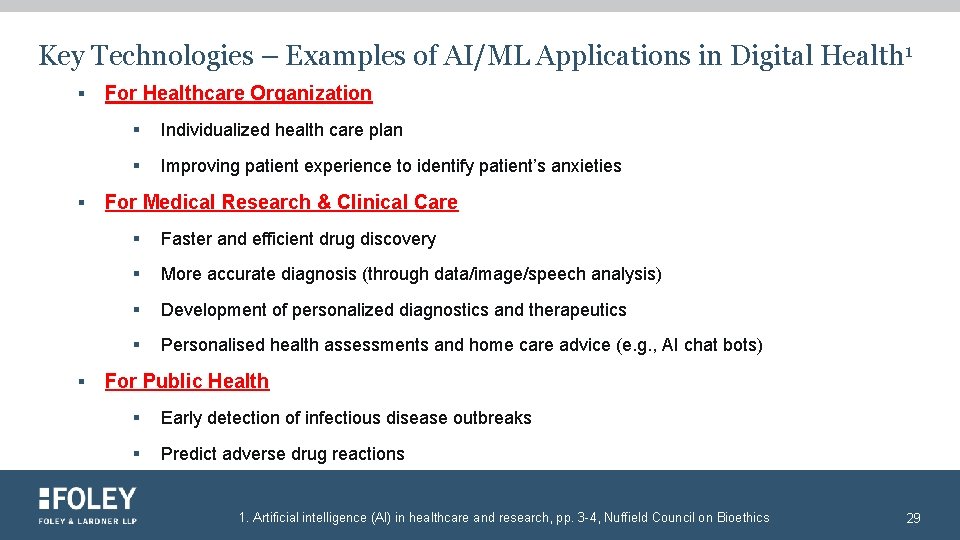
Key Technologies – Examples of AI/ML Applications in Digital Health 1 § § § For Healthcare Organization § Individualized health care plan § Improving patient experience to identify patient’s anxieties For Medical Research & Clinical Care § Faster and efficient drug discovery § More accurate diagnosis (through data/image/speech analysis) § Development of personalized diagnostics and therapeutics § Personalised health assessments and home care advice (e. g. , AI chat bots) For Public Health § Early detection of infectious disease outbreaks § Predict adverse drug reactions 1. Artificial intelligence (AI) in healthcare and research, pp. 3 -4, Nuffield Council on Bioethics 29

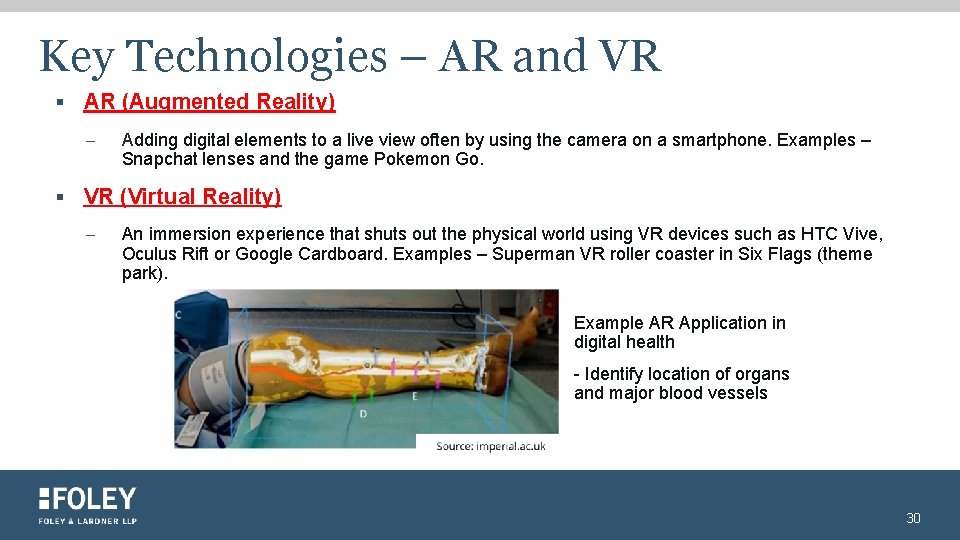
Key Technologies – AR and VR § AR (Augmented Reality) – Adding digital elements to a live view often by using the camera on a smartphone. Examples – Snapchat lenses and the game Pokemon Go. § VR (Virtual Reality) – An immersion experience that shuts out the physical world using VR devices such as HTC Vive, Oculus Rift or Google Cardboard. Examples – Superman VR roller coaster in Six Flags (theme park). Example AR Application in digital health - Identify location of organs and major blood vessels 30

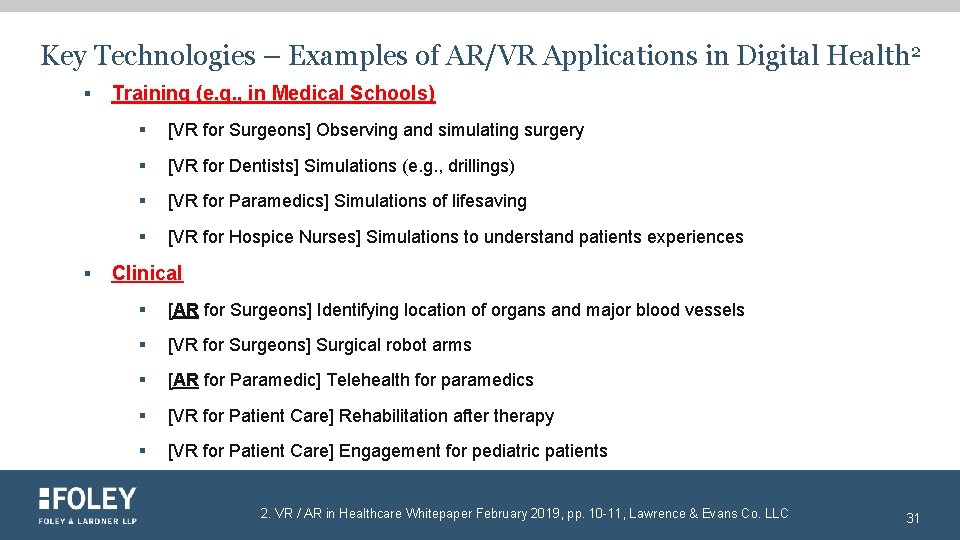
Key Technologies – Examples of AR/VR Applications in Digital Health 2 § § Training (e. g. , in Medical Schools) § [VR for Surgeons] Observing and simulating surgery § [VR for Dentists] Simulations (e. g. , drillings) § [VR for Paramedics] Simulations of lifesaving § [VR for Hospice Nurses] Simulations to understand patients experiences Clinical § [AR for Surgeons] Identifying location of organs and major blood vessels § [VR for Surgeons] Surgical robot arms § [AR for Paramedic] Telehealth for paramedics § [VR for Patient Care] Rehabilitation after therapy § [VR for Patient Care] Engagement for pediatric patients 2. VR / AR in Healthcare Whitepaper February 2019, pp. 10 -11, Lawrence & Evans Co. LLC 31

Key Technologies – Blockchain § Blockchain – A cryptography-based distributed ledger system that can record valid transactions between parties across a peer-to-peer network of personal computers without the need of a trusted authority or central server. § Benefits of Blockchain to Healthcare Applications 3 – Decentralization – stakeholders access health data without central authority – Improved data security and privacy of patients data – through strong cryptographic protocols and well -defined smart contracts. – Availability and Robustness – through data duplicated in multiple nodes – Transparency and Trust – open and transparent nature creates trust across distributed healthcare applications – Data Verifiability - through cryptographic protocols; useful in the area of pharmaceutical supply chain management and insurance claim processing 3. Cornelius C. Agbo, Qusay H. Mahmoud and J. Mikael Eklund, "Blockchain Technology in Healthcare: Systematic Review", p. 7, Healthcare 2019 32

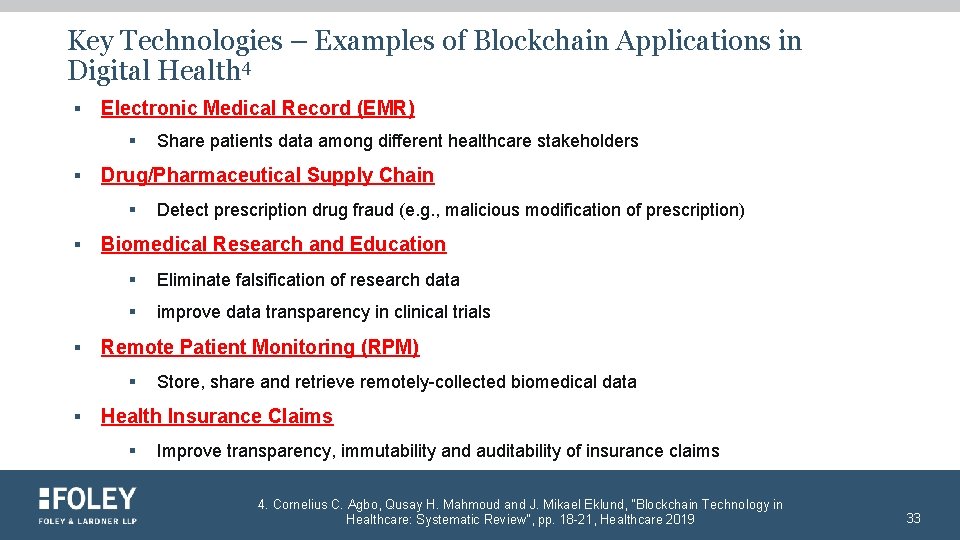
Key Technologies – Examples of Blockchain Applications in Digital Health 4 § Electronic Medical Record (EMR) § § Drug/Pharmaceutical Supply Chain § § § Detect prescription drug fraud (e. g. , malicious modification of prescription) Biomedical Research and Education § Eliminate falsification of research data § improve data transparency in clinical trials Remote Patient Monitoring (RPM) § § Share patients data among different healthcare stakeholders Store, share and retrieve remotely-collected biomedical data Health Insurance Claims § Improve transparency, immutability and auditability of insurance claims 4. Cornelius C. Agbo, Qusay H. Mahmoud and J. Mikael Eklund, "Blockchain Technology in Healthcare: Systematic Review", pp. 18 -21, Healthcare 2019 33

Key Legal Issues – Cybersecurity and Privacy - What is Reasonable Security? § Unfortunately, there is no uniform standard across industries § Really, it depends on your industry, information assets, services and products, and risk tolerance. For guidance, look to: – Laws, Frameworks, and Regulatory Guidance – Security Frameworks/Technical Controls – Risk Assessment Findings – Case Law and Enforcement Actions 34

General Data Protection Regulation (GDPR) (Europe) § Effective date: May 25, 2018 § Applies to the processing of “personal data” § Applicable to organizations that: – Maintain an establishment in the EU – Engage in processing activities related to the offering of goods or services to data subjects in the EU – Engage in processing activities related to monitoring the behavior of data subjects while in the EU

GDPR (continued) § Controllers and processors § Data Protection Officers (DPO) § Breach obligations § Data subjects have additional rights regarding their data (e. g. , right to access, restrict, be forgotten, etc. ) § Penalty for non-compliance: fines of up to 4% of global revenue or EUR 20 million, whichever is higher 36

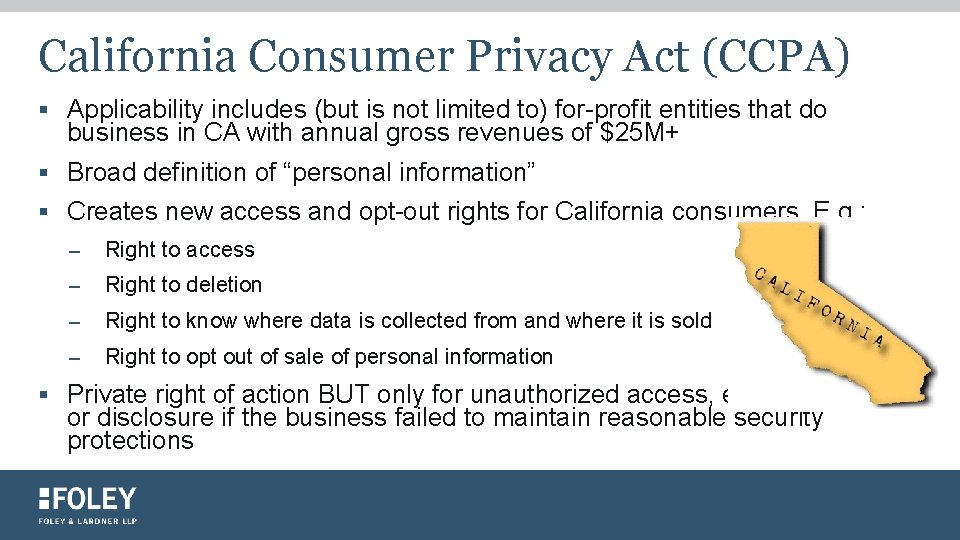
California Consumer Privacy Act (CCPA) § Applicability includes (but is not limited to) for-profit entities that do business in CA with annual gross revenues of $25 M+ § Broad definition of “personal information” § Creates new access and opt-out rights for California consumers. E. g. : – Right to access – Right to deletion – Right to know where data is collected from and where it is sold – Right to opt out of sale of personal information § Private right of action BUT only for unauthorized access, exfiltration, theft, or disclosure if the business failed to maintain reasonable security protections

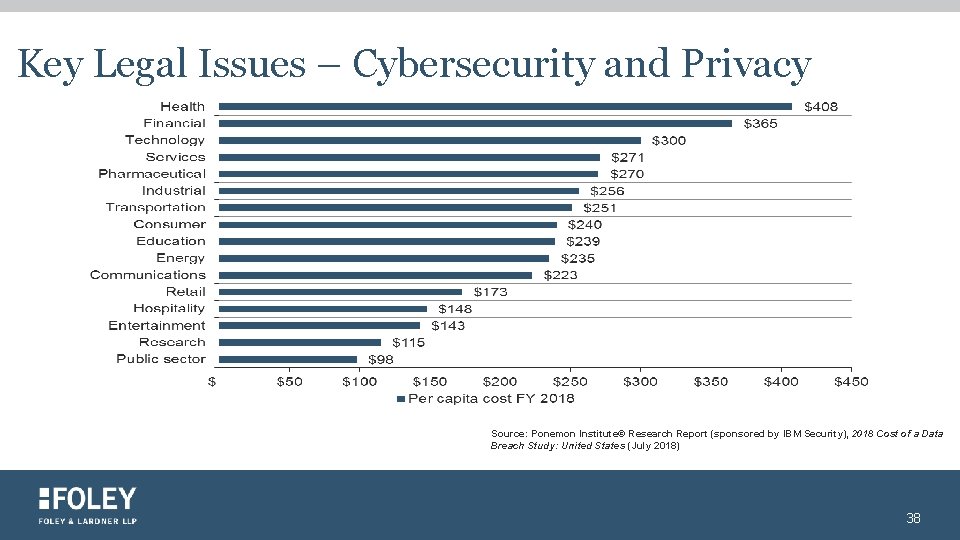
Key Legal Issues – Cybersecurity and Privacy Source: Ponemon Institute© Research Report (sponsored by IBM Security), 2018 Cost of a Data Breach Study: United States (July 2018) 38

Key Legal Issues – Patent Protection § Patent Eligibility: Various countries have different approaches for protecting software – easier to gain such protection in some countries versus others § Consider solutions that cross borders (e. g. , software updates from a server located in a different country) § Protecting individual component versus entire system solution of wearable devices having specific programming that communicate with a base platform 39

Key Legal Issues – Copyright Protection § Copyright protection? – Protect collection/presentation of data, but usually not on the underlying raw data § Consider protection for databases: – As compilations of data, but consider scope of protection – Database Directive in EU 40

Key Legal Issues – Trade Secret Protection § Trade secret information is: (1) information (many kinds qualify); (2) that derives economic value (actual or potential) because it is not generally known; and (3) that is the subject of reasonable efforts to maintain its secrecy. § Examples: Market predictions, customer lists, business strategy, computer algorithms, compiled data sets – Consider areas where other forms of protection, such as patent protection, are not available 41

Key Legal Issues – Trade Secret Protection § U. S. : Laws provide trade secret owners with the ability to choose between United State federal and state court forums for trade secret misappropriation. Defend Trade Secrets Act of 2016 (U. S. Federal law) 42

Key Legal Issues – Indemnification § Indemnification, i. e. , who takes responsibility such as to third parties and for breaches – Patent – Copyright – Open source/proprietary – Trade secret breach – Non-intellectual property, such as confidentiality, product liability, etc. 43

Thank You! Questions? Please contact: Kristel Schorr – kschorr@foley. com Pavan Agarwal - pagarwal@foley. com Jihwang Yeo – jyeo@foley. com ATTORNEY ADVERTISEMENT. The contents of this document, current at the date of publication, are for reference purposes only and do not constitute legal advice. Where previous cases are included, prior results do not guarantee a similar outcome. Images of people may not be Foley personnel. © 2018 Foley & Lardner LLP * With thanks to Jennifer Rathburn for input on cybersecurity/privacy
 Kristel schorr
Kristel schorr Pavan kumar vijay
Pavan kumar vijay Pavan kumar vijay
Pavan kumar vijay Pavan jayaraman
Pavan jayaraman Jokars
Jokars Pavan sukhdev wwf
Pavan sukhdev wwf Pavan uppal
Pavan uppal Pavan piratla
Pavan piratla Kristel reim
Kristel reim Reaktsiooninorm
Reaktsiooninorm Kristel ojala
Kristel ojala Daltonism pärilik
Daltonism pärilik Kristina ojuland
Kristina ojuland Kristel kivinurm-priisalm
Kristel kivinurm-priisalm Kristel tamm
Kristel tamm Kristel toom
Kristel toom Kristel ojala
Kristel ojala Kristel järve
Kristel järve Kristel uiboaed
Kristel uiboaed Kristel johanson
Kristel johanson Kristel kilk
Kristel kilk Kristel tuhk
Kristel tuhk Kristel tamm
Kristel tamm Replikatsioon ja transkriptsioon
Replikatsioon ja transkriptsioon Adria junyent-ferre
Adria junyent-ferre Kristel tamm
Kristel tamm Kristel ojala
Kristel ojala Sundarlal bahuguna quotes
Sundarlal bahuguna quotes Shweta agarwal iit madras
Shweta agarwal iit madras Coen 311 concordia
Coen 311 concordia Amit agarwal princeton
Amit agarwal princeton Dr jyotsna agarwal
Dr jyotsna agarwal Ankit agarwal coinbase
Ankit agarwal coinbase Neera agarwal
Neera agarwal Petrolera zuata
Petrolera zuata Abhinav agarwal stanford
Abhinav agarwal stanford Chhavi agarwal md
Chhavi agarwal md Abhinav agarwal stanford
Abhinav agarwal stanford Sita speak by bina agarwal
Sita speak by bina agarwal Avinash agarwal
Avinash agarwal Vasudha agarwal
Vasudha agarwal Ravi agarwal md
Ravi agarwal md Microsoft decision service
Microsoft decision service Yuvraj agarwal ucsd
Yuvraj agarwal ucsd Dr shaleen agarwal
Dr shaleen agarwal