AOSC 620 Ozone in the Stratosphere R Dickerson









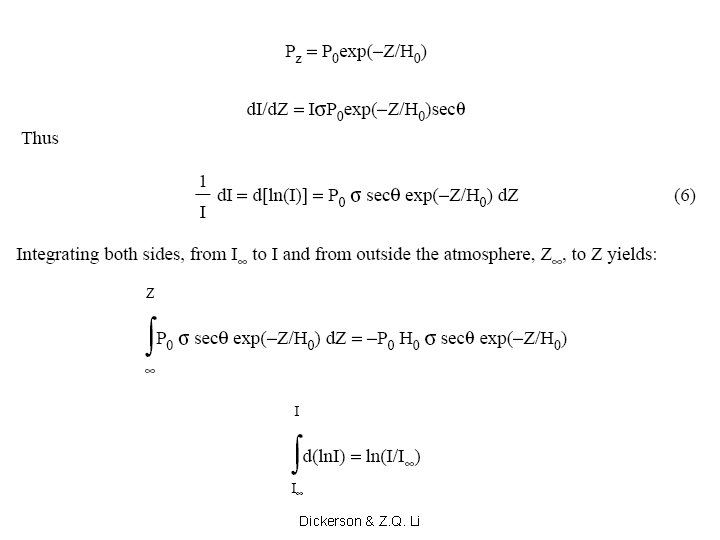




- Slides: 38

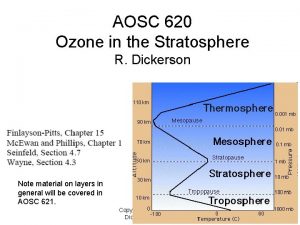
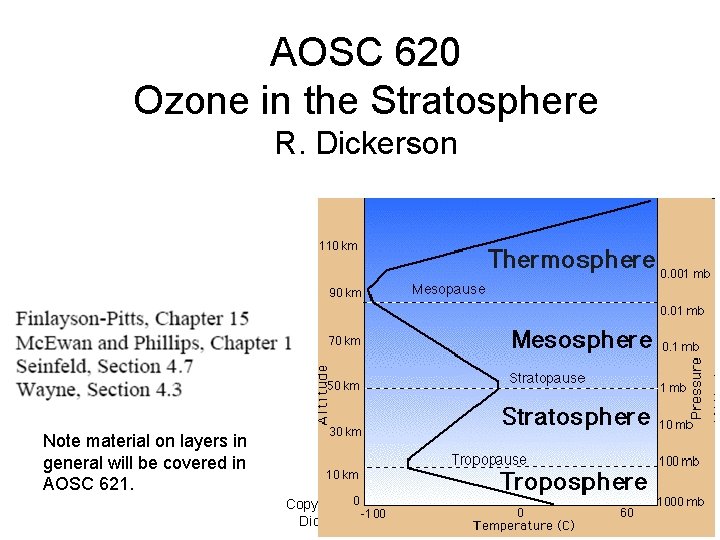
AOSC 620 Ozone in the Stratosphere R. Dickerson Note material on layers in general will be covered in AOSC 621. Copyright © 2010 R. R. Dickerson & Z. Q. Li 1

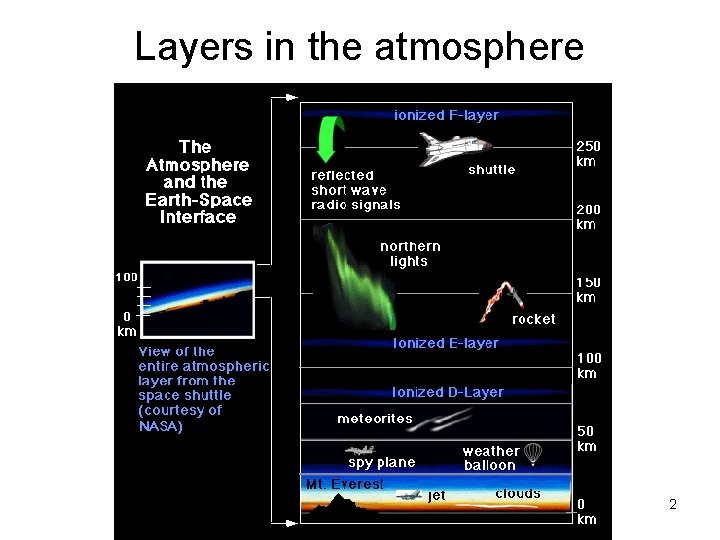
Layers in the atmosphere Copyright © 2013 R. R. Dickerson & Z. Q. Li 2

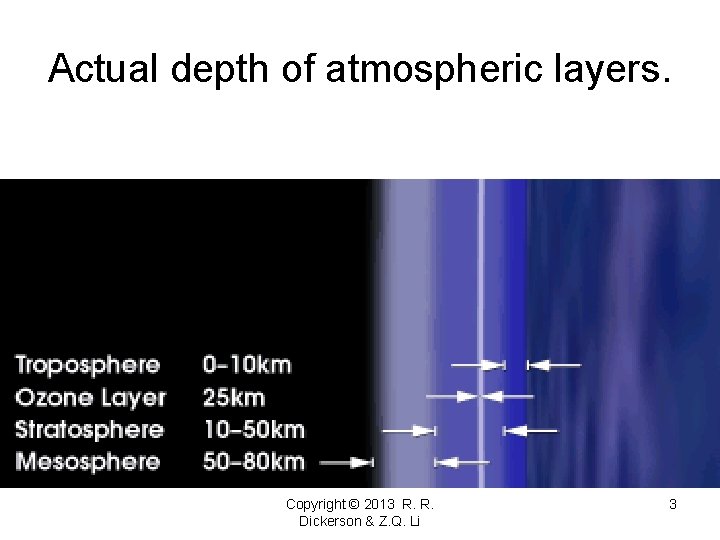
Actual depth of atmospheric layers. Copyright © 2013 R. R. Dickerson & Z. Q. Li 3

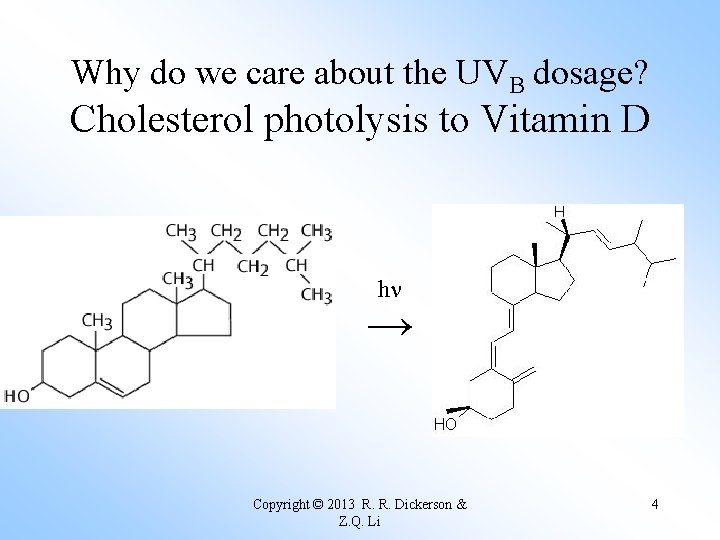
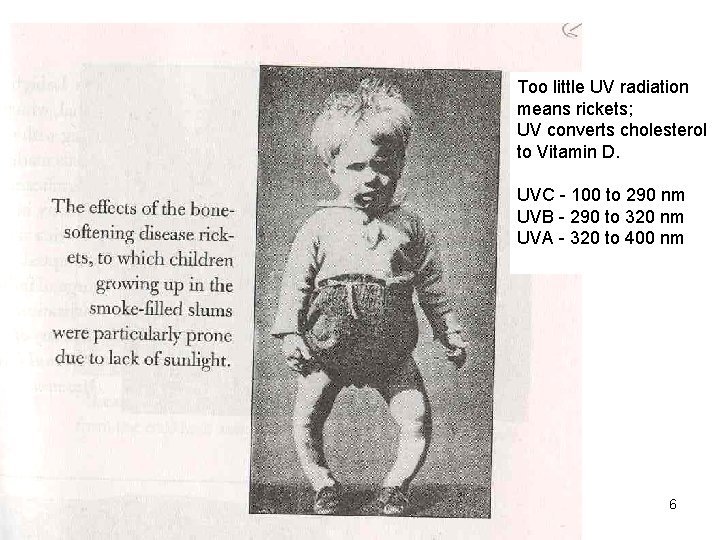
Why do we care about the UVB dosage? Cholesterol photolysis to Vitamin D hn → Copyright © 2013 R. R. Dickerson & Z. Q. Li 4

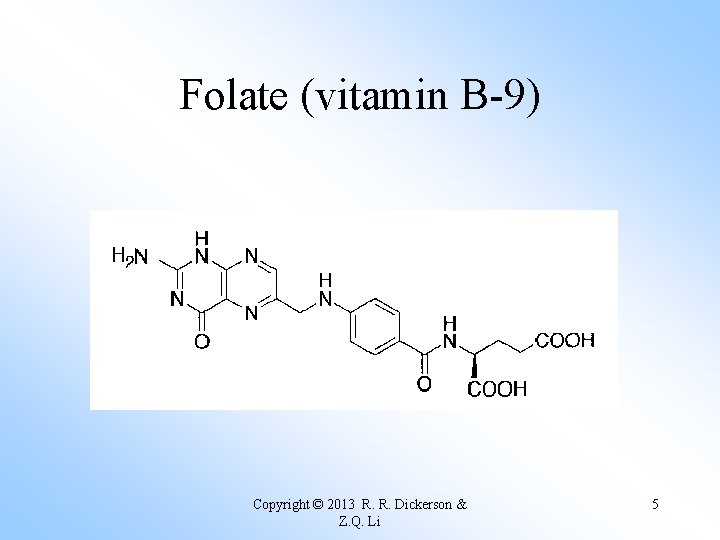
Folate (vitamin B-9) Copyright © 2013 R. R. Dickerson & Z. Q. Li 5

Too little UV radiation means rickets; UV converts cholesterol to Vitamin D. UVC - 100 to 290 nm UVB - 290 to 320 nm UVA - 320 to 400 nm Copyright © 2013 R. R. Dickerson & Z. Q. Li 6

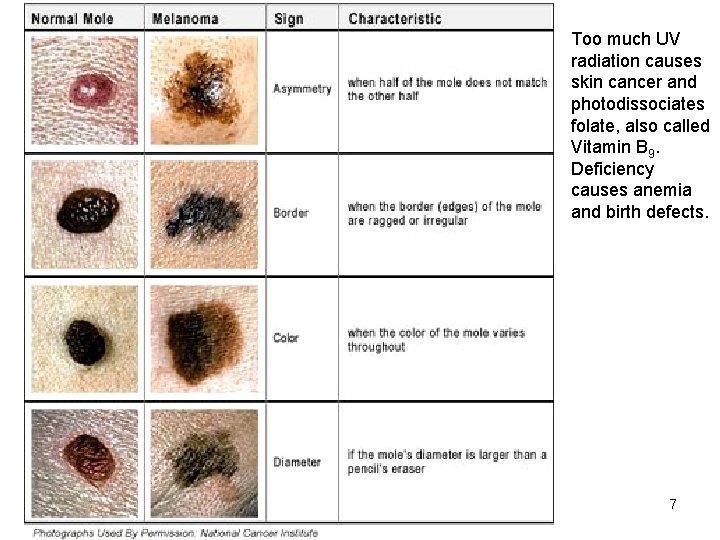
Too much UV radiation causes skin cancer and photodissociates folate, also called Vitamin B 9. Deficiency causes anemia and birth defects. Copyright © 2013 R. R. Dickerson & Z. Q. Li 7

Copyright © 2013 R. R. Dickerson & Z. Q. Li 8

Copyright © 2013 R. R. Dickerson & Z. Q. Li 9

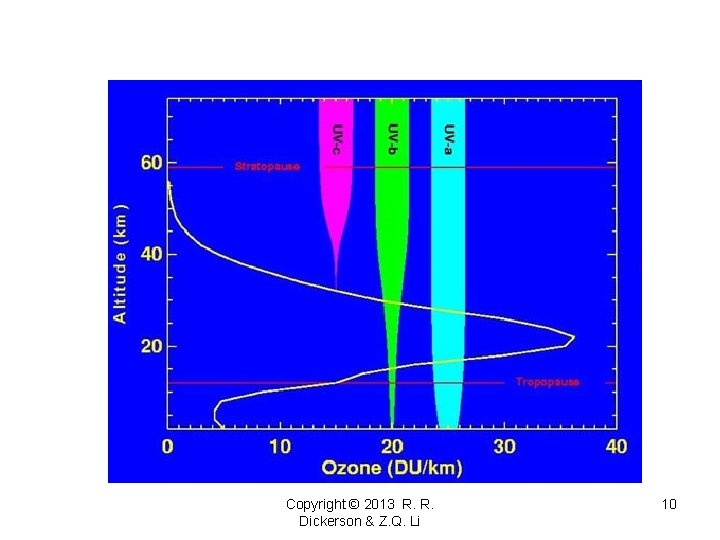
Copyright © 2013 R. R. Dickerson & Z. Q. Li 10

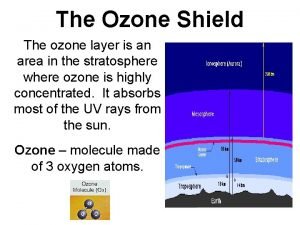
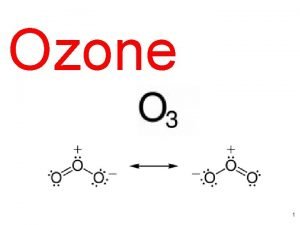
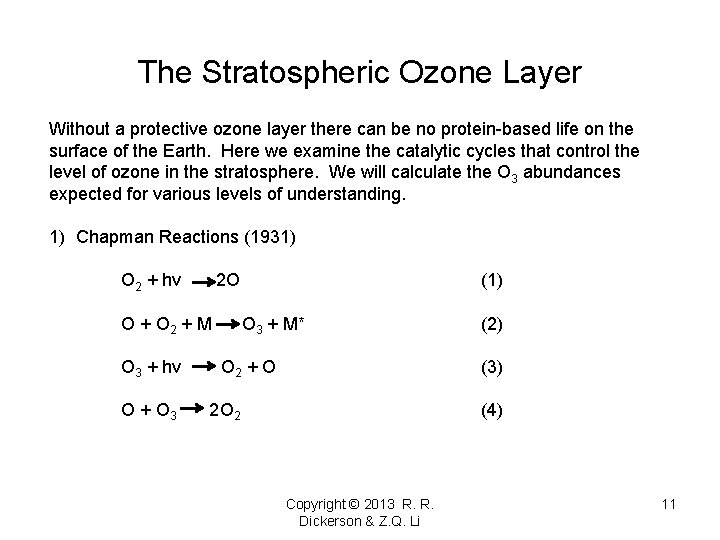
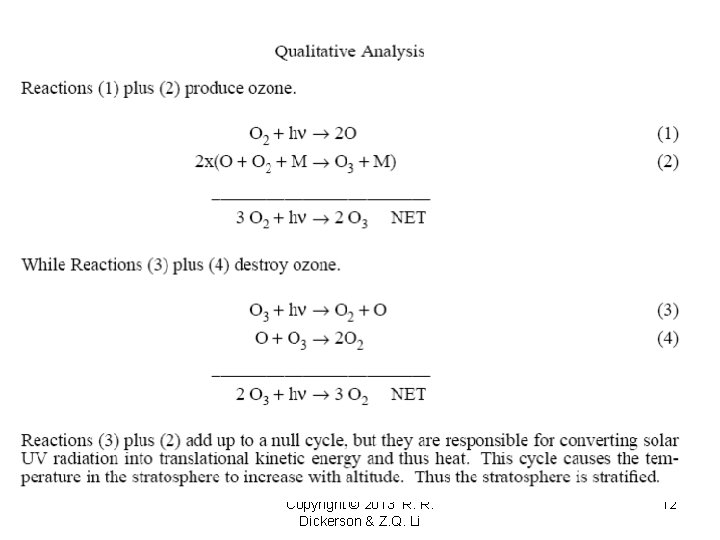
The Stratospheric Ozone Layer Without a protective ozone layer there can be no protein-based life on the surface of the Earth. Here we examine the catalytic cycles that control the level of ozone in the stratosphere. We will calculate the O 3 abundances expected for various levels of understanding. 1) Chapman Reactions (1931) O 2 + hv 2 O O + O 2 + M O 3 + hv O + O 3 (1) O 3 + M* O 2 + O (2) (3) 2 O 2 (4) Copyright © 2013 R. R. Dickerson & Z. Q. Li 11

Copyright © 2013 R. R. Dickerson & Z. Q. Li 12

Copyright © 2013 R. R. Dickerson & Z. Q. Li 13

Copyright © 2013 R. R. Dickerson & Z. Q. Li 14

Copyright © 2013 R. R. Dickerson & Z. Q. Li 15

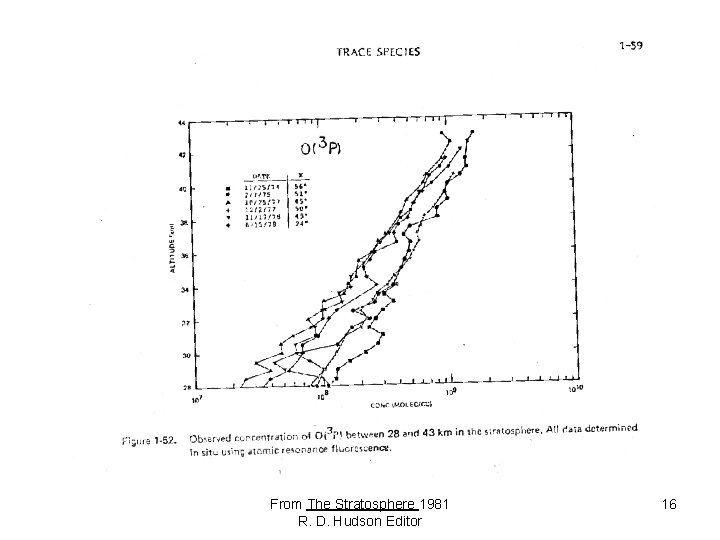
From The Stratosphere 1981 R. D. Hudson Editor 16

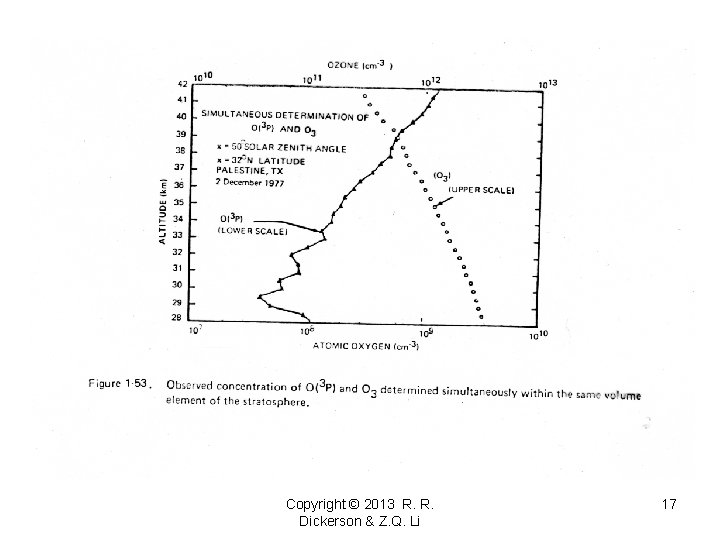
Copyright © 2013 R. R. Dickerson & Z. Q. Li 17

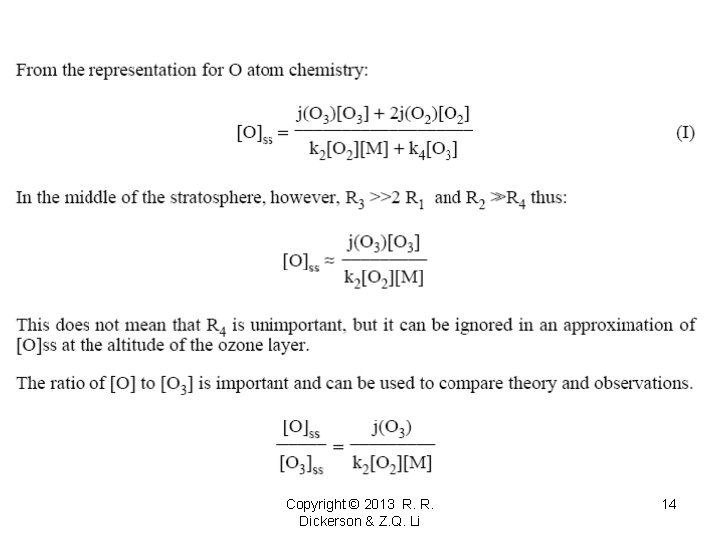
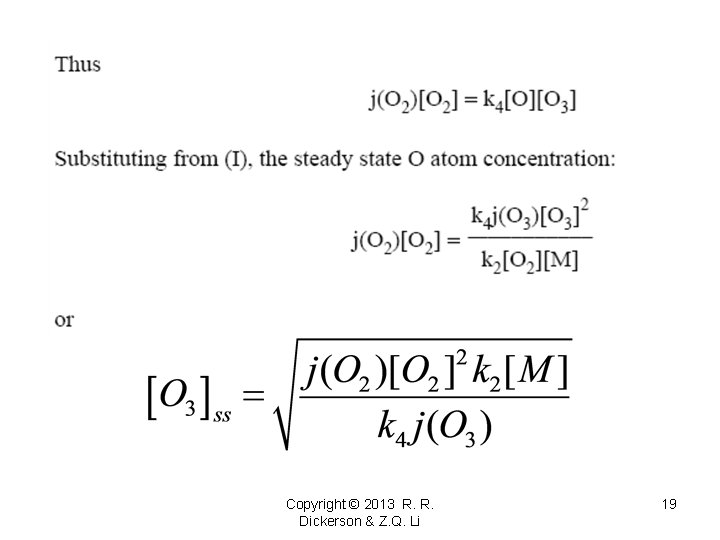
O/O 3 = j(O 3)/(k 2 M O 2) Copyright © 2013 R. R. Dickerson & Z. Q. Li 18

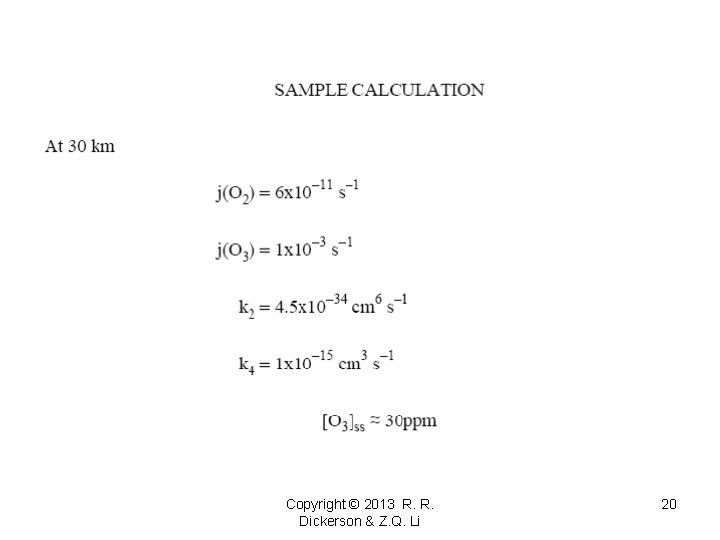
Copyright © 2013 R. R. Dickerson & Z. Q. Li 19

Copyright © 2013 R. R. Dickerson & Z. Q. Li 20

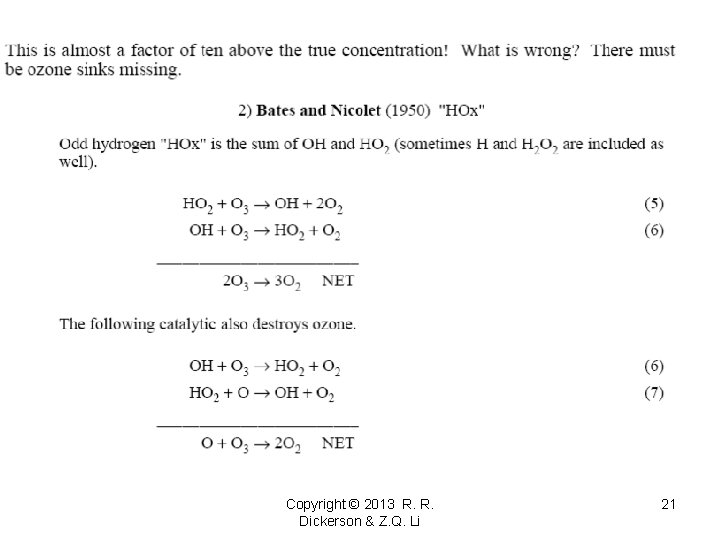
Copyright © 2013 R. R. Dickerson & Z. Q. Li 21

Copyright © 2013 R. R. Dickerson & Z. Q. Li 22

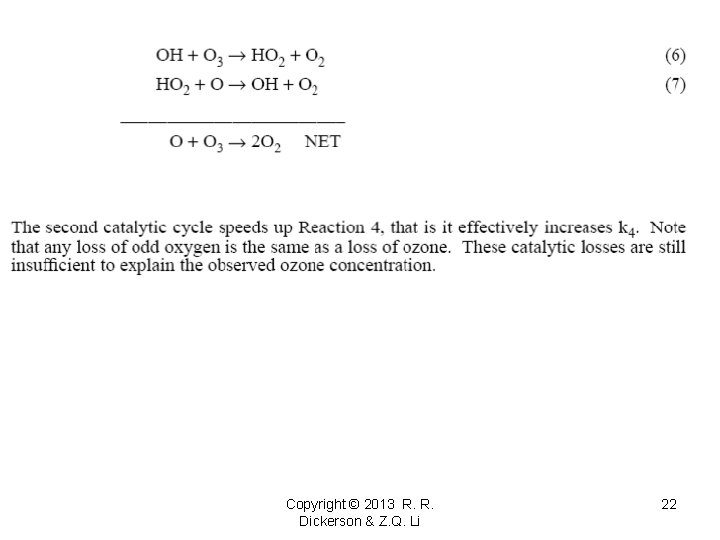
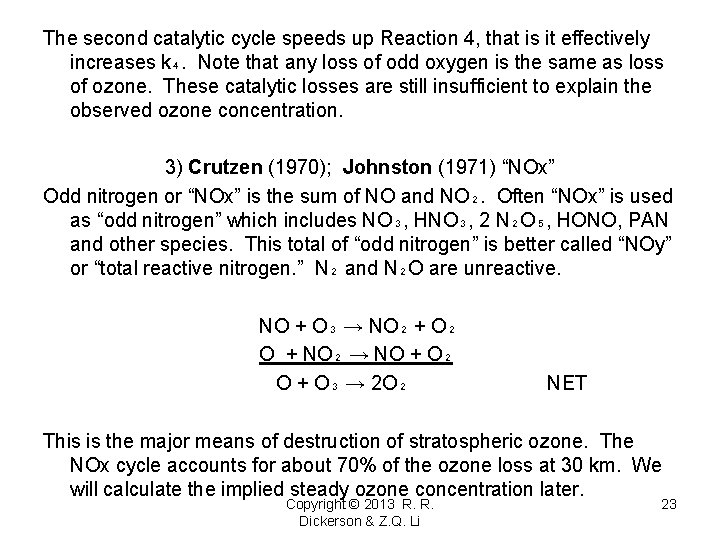
The second catalytic cycle speeds up Reaction 4, that is it effectively increases k₄. Note that any loss of odd oxygen is the same as loss of ozone. These catalytic losses are still insufficient to explain the observed ozone concentration. 3) Crutzen (1970); Johnston (1971) “NOx” Odd nitrogen or “NOx” is the sum of NO and NO₂. Often “NOx” is used as “odd nitrogen” which includes NO₃, HNO₃, 2 N₂O₅, HONO, PAN and other species. This total of “odd nitrogen” is better called “NOy” or “total reactive nitrogen. ” N₂ and N₂O are unreactive. NO + O₃ → NO₂ + O₂ O + NO₂ → NO + O₂ O + O₃ → 2 O₂ NET This is the major means of destruction of stratospheric ozone. The NOx cycle accounts for about 70% of the ozone loss at 30 km. We will calculate the implied steady ozone concentration later. Copyright © 2013 R. R. Dickerson & Z. Q. Li 23

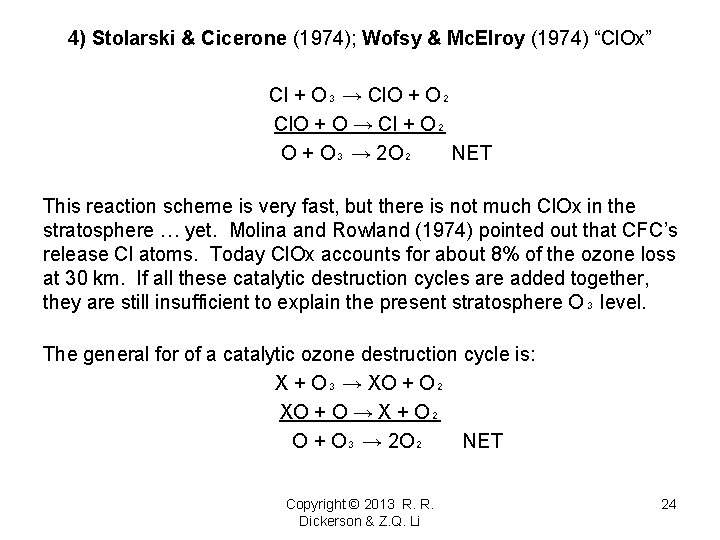
4) Stolarski & Cicerone (1974); Wofsy & Mc. Elroy (1974) “Cl. Ox” Cl + O₃ → Cl. O + O₂ Cl. O + O → Cl + O₂ O + O₃ → 2 O₂ NET This reaction scheme is very fast, but there is not much Cl. Ox in the stratosphere … yet. Molina and Rowland (1974) pointed out that CFC’s release Cl atoms. Today Cl. Ox accounts for about 8% of the ozone loss at 30 km. If all these catalytic destruction cycles are added together, they are still insufficient to explain the present stratosphere O₃ level. The general for of a catalytic ozone destruction cycle is: X + O₃ → XO + O₂ XO + O → X + O₂ O + O₃ → 2 O₂ NET Copyright © 2013 R. R. Dickerson & Z. Q. Li 24

The Stratospheric Ozone Layer Copyright © 2013 R. R. Dickerson & Z. Q. Li 25

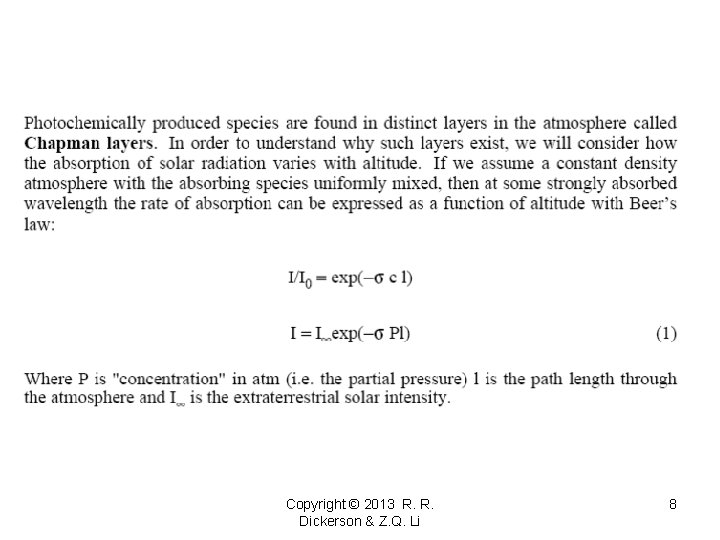
How did Farby and Buisson do? At 3000 Å (300 nm) 1/100 = I/Io = ln(-ecl) e 300 = 9. 5 atm-1 cm-1 -ln(0. 01)/9. 5 = 0. 485 atm cm (compare to 0. 50) 0. 485 atm cm = 485 DU Correct for zenith angle of 27 o for Paris? (cos 27 o = 0. 9 so W = 432 DU. ) [O 3] = 0. 6 cm 3 m-3 = 6 x 10 -7 Is that right? [O 3] for uniform mixing ratio = column content/scale height 5 mm/8 x 106 mm = 6. 25 x 10– 7 = 625 ppb As Fabry said this is too much for surface air, and the ozone layer lies above. Copyright © 2013 R. R. Dickerson & Z. Q. Li 26

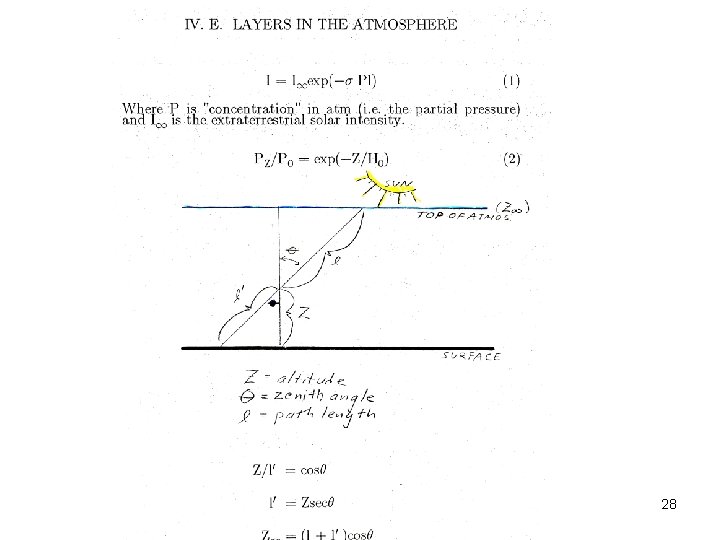
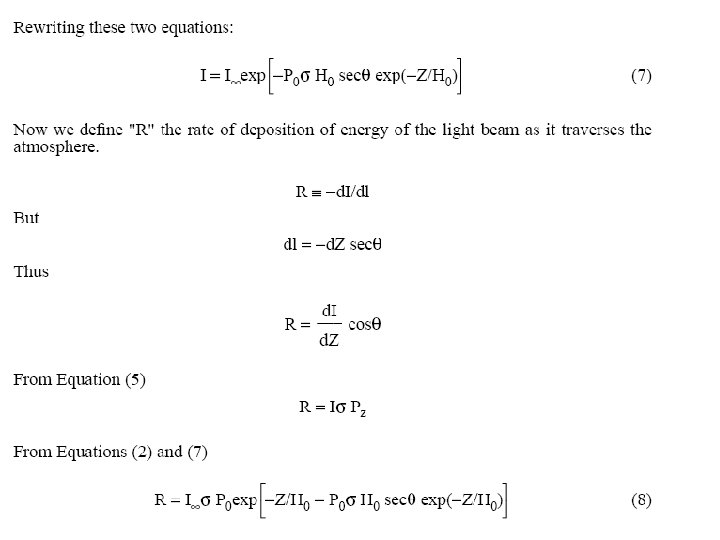
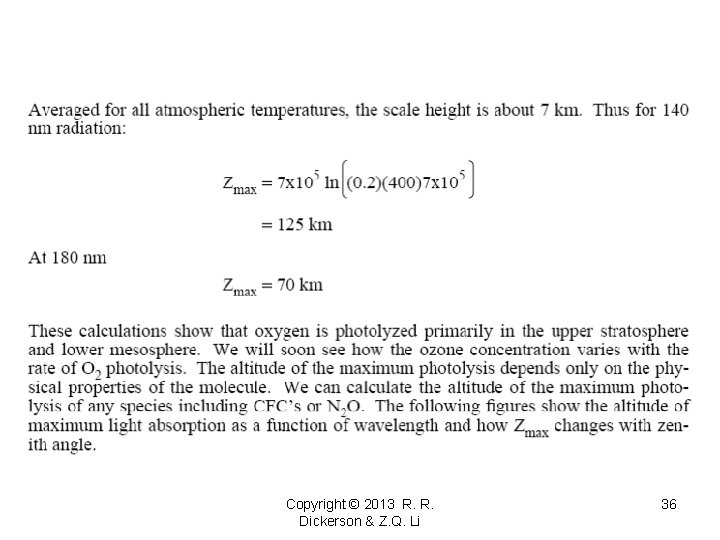
Slides 27 on will be presented in AOSC 621 in 2015. Copyright © 2010 R. R. Dickerson & Z. Q. Li 27

Copyright © 2010 R. R. Dickerson & Z. Q. Li 28

Copyright © 2010 R. R. Dickerson & Z. Q. Li 29

Copyright © 2010 R. R. Dickerson & Z. Q. Li 30
Copyright © 2010 R. R. Dickerson & Z. Q. Li 31

Copyright © 2010 R. R. Dickerson & Z. Q. Li 32

Copyright © 2010 R. R. Dickerson & Z. Q. Li 33

Copyright © 2013 R. R. Dickerson & Z. Q. Li 34

Copyright © 2013 R. R. Dickerson & Z. Q. Li 35

Copyright © 2013 R. R. Dickerson & Z. Q. Li 36

Copyright © 2013 R. R. Dickerson & Z. Q. Li 37

Copyright © 2010 R. R. Dickerson & Z. Q. Li 38
 Cmsc 320 github
Cmsc 320 github Evan dickerson
Evan dickerson John p dickerson
John p dickerson Sally dickerson
Sally dickerson How cold is the stratosphere
How cold is the stratosphere Drag
Drag Stratosphere
Stratosphere Layer closest to earth
Layer closest to earth Stratosphere altitude
Stratosphere altitude Here is where your presentation begins artinya
Here is where your presentation begins artinya Krs 620
Krs 620 Schedule 1 tdg
Schedule 1 tdg Aruba mesh ap
Aruba mesh ap Sy 211
Sy 211 Emc data domain 620
Emc data domain 620 12vac35-105-160
12vac35-105-160 Measuring some 620 by 513 feet
Measuring some 620 by 513 feet Jms 620
Jms 620 Acars taxi
Acars taxi Toefl 勉強
Toefl 勉強 Isa 620 using the work of an expert
Isa 620 using the work of an expert Meam 620
Meam 620 Wheres the ozone layer
Wheres the ozone layer Ozone layer levels
Ozone layer levels How is total ozone distributed over the globe
How is total ozone distributed over the globe Protection of ozone layer
Protection of ozone layer Ozone molecular geometry
Ozone molecular geometry Sulphur oxide
Sulphur oxide Ozone solid state
Ozone solid state Ozone without borders
Ozone without borders Technic
Technic Drawing lewis structures practice
Drawing lewis structures practice Ozone layer depletion
Ozone layer depletion Effect of ozone depletion on plants
Effect of ozone depletion on plants Ozone nasa
Ozone nasa Vray sun ozone
Vray sun ozone P
P Methyl nitrite lewis structure
Methyl nitrite lewis structure Sopmed ozone
Sopmed ozone