Atmospheric Processes Ozone Depletion Quick recap The ozone












- Slides: 24

Atmospheric Processes Ozone Depletion

Quick recap… • "The ozone layer" refers to the ozone within stratosphere • over 90% of the earth's ozone resides • Ozone is an irritating, corrosive, colourless gas with a smell • O 3 formed by Ozone-Oxygen cycle, UV catalyst • A reduction in atmospheric ozone has been observed in two ways – General global reduction rate of approx. 4% – Polar ‘ozone holes’ – annual drop in stratospheric concentration. Up to ⅔ over the Antarctic and ⅓ over the Arctic

Ozone depletion history… • Satellite measurements of ozone started in the early 70's • first comprehensive worldwide measurements started in 1978 with the Nimbus -7 satellite – carried TOMS (total ozone mapping spectrometer) • today there are several different satellites measuring concentrations of ozone and other atmospheric gases • Chlorofluorocarbons were first created in 1928 as non-toxic, non-flamable refrigerants, and were first produced commercially in the 1930's by Du. Pont • 1974 – research showed a link between CFCs and ozone depletion – predicted a 7% decrease in 60 years • US banned CFC's in aerosol sprays in 1978 • Data collected by the British Antarctic Survey showing that ozone levels had dropped to 10% below normal January levels for Antarctica. • NASA soon discovered that the spring-time ''ozone hole'' had been covered up by a computer-program designed to discard sudden, large drops in ozone concentrations as ''errors'‘ • Numerous studies since then have confirmed both the Antarctic hole, as well as an overall global decrease in Ozone • One major study calculates that the global ozone has decreased 2. 5% from 1969 to 1986 and another 3% drop from 1986 to 1993

• http: //www. youtube. com/watch? v=q. Uf. VMog Idr 8&feature=player_embedded • http: //www. nasa. gov/topics/earth/features/ world_avoided. html

Why is there concern about ozone depletion? If UVB reaches the Earth’s surface it could; Be absorbed by living cells, which can: • break up biological molecules • Causing damage to DNA – food chain implications • Causes cancers or possibly cataracts • Damage to plant tissue • Damage to marine plankton

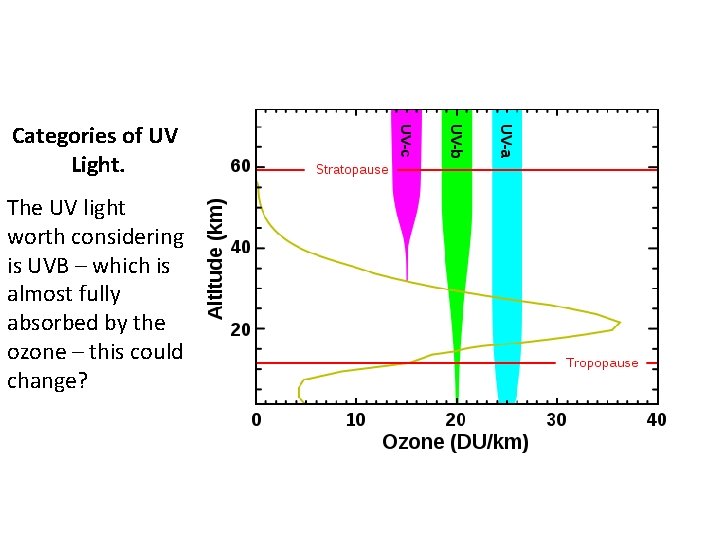
Categories of UV Light. The UV light worth considering is UVB – which is almost fully absorbed by the ozone – this could change?

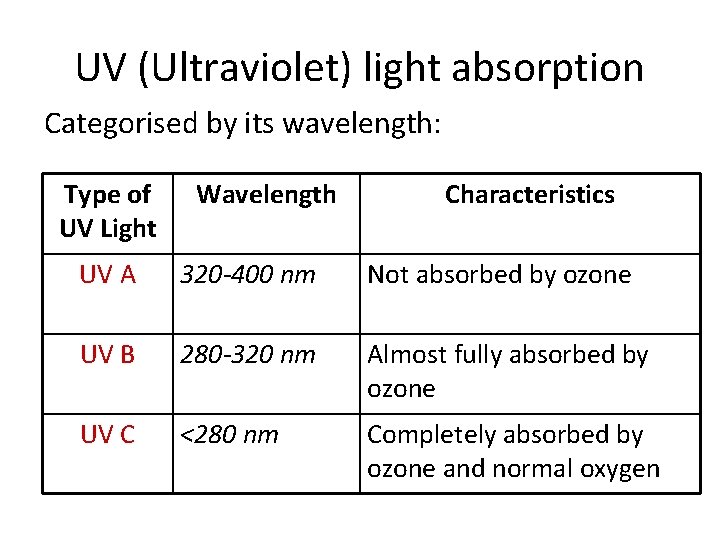
UV (Ultraviolet) light absorption Categorised by its wavelength: Type of UV Light Wavelength Characteristics UV A 320 -400 nm Not absorbed by ozone UV B 280 -320 nm Almost fully absorbed by ozone UV C <280 nm Completely absorbed by ozone and normal oxygen

The effects of UVB on gases in the atmosphere • Number of chemical reactions • Very little reaches the Earth’s surface • Diatomic, Triatomic and photolytic reactions where molecules are split (e. g. Oxygen-Ozone Cycle) Summary of reactions; UV O 3 O 2 + O UV

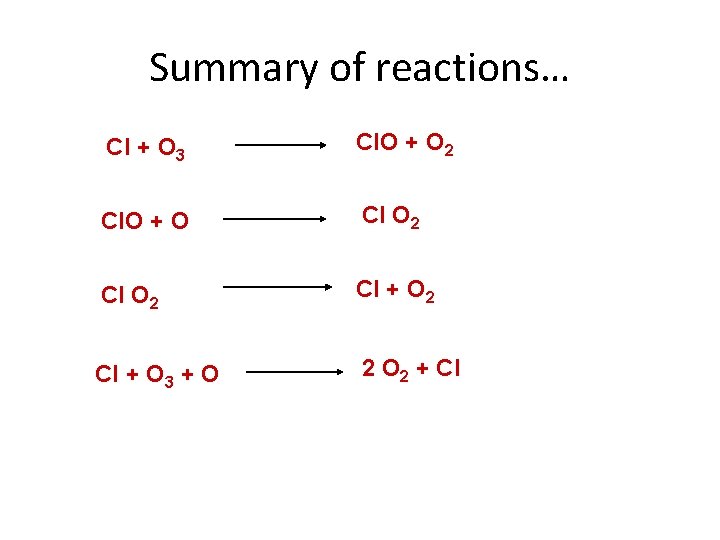
Chlorine and ozone depletion • A halogen – involved in a range of reactions • Radical – atomic state, unpaired, very reactive • Reacts with O 3, breaking it down leaving O and Cl. O (Chlorine Monoxide) • Reacts again with O to make Cl. O 2 • Reacts again releasing Cl (occurs thousands of times) • Finishing with a free Chlorine radical, thus removing important materials to maintain the dynamic equilibrium which is the ozone layer

Summary of reactions… Cl + O 3 Cl. O + O 2 Cl. O + O Cl O 2 Cl + O 3 + O 2 + Cl

Pollution by CFCs (Chlorofluorocarbons) • • Chlorine – Fluorine – Carbon Developed as coolants for refrigerators in the 1920 s replacing ammonia (NH 3) – low boiling point, non-toxic and generally non reactive Later used for aerosols, propellants, solvents, circuit boards, packing materials and expanding foams Chemically very stable and low solubility – long residence time in the atmosphere (potential to be carried to the stratosphere) CFCs do not reduce ozone – it is the breakdown and subsequent release of chlorine that causes the damage

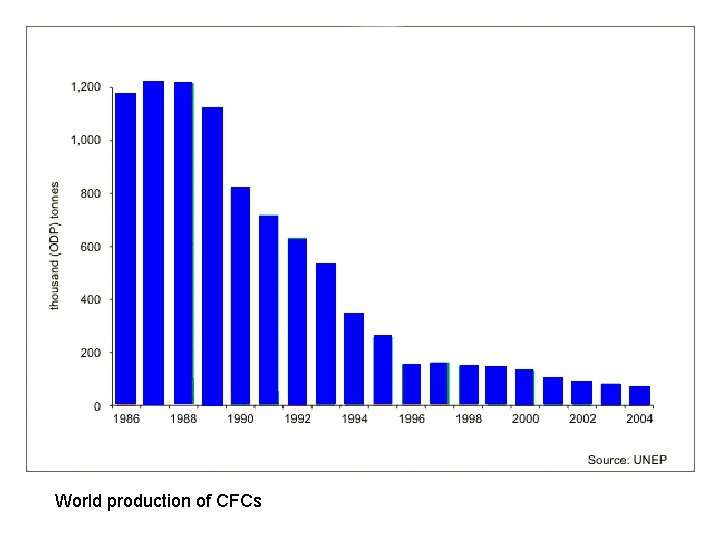
World production of CFCs

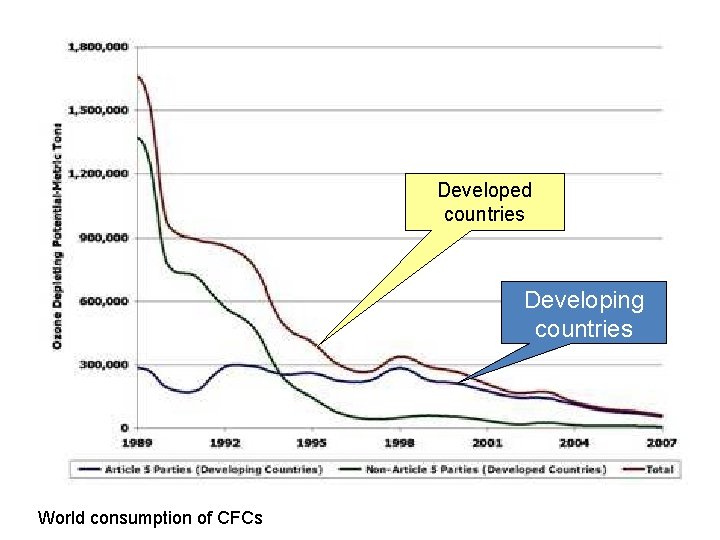
Developed countries Developing countries World consumption of CFCs

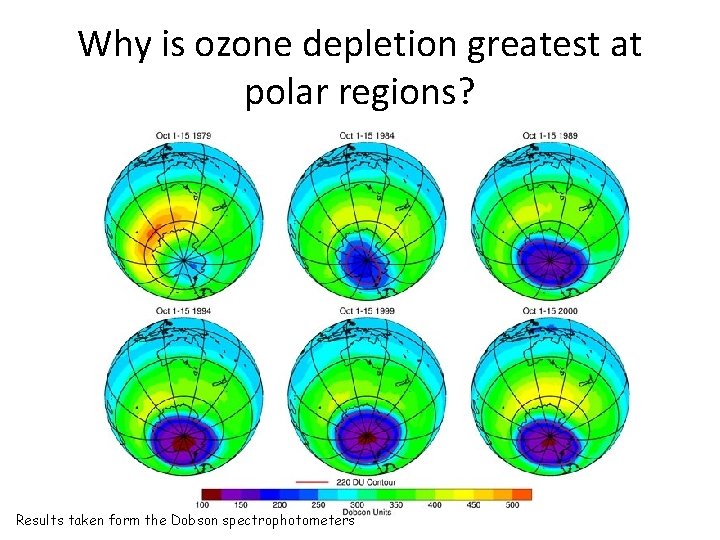
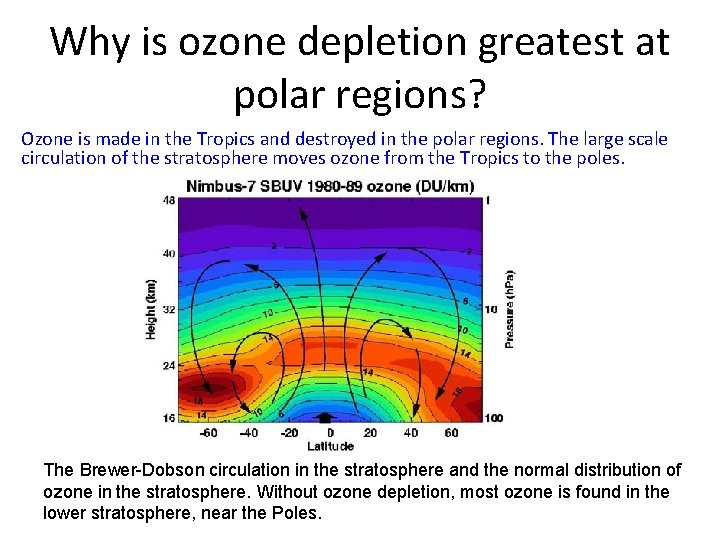
Why is ozone depletion greatest at polar regions? Results taken form the Dobson spectrophotometers

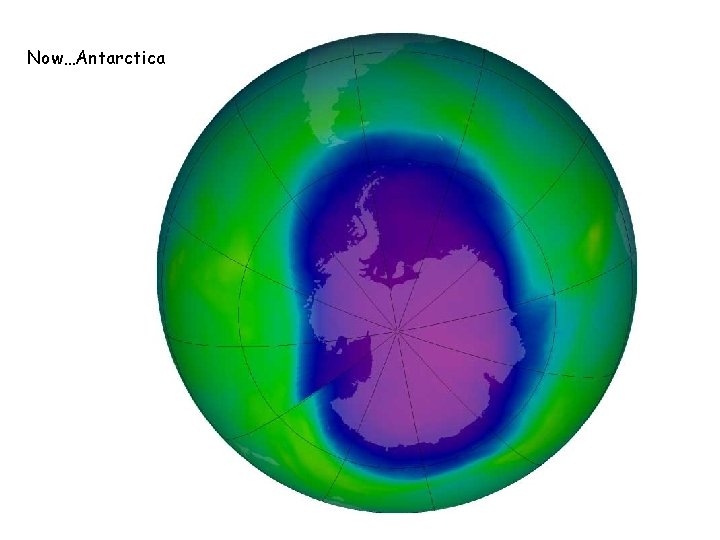
Now…Antarctica

Why is ozone depletion greatest at polar regions? Ozone is made in the Tropics and destroyed in the polar regions. The large scale circulation of the stratosphere moves ozone from the Tropics to the poles. The Brewer-Dobson circulation in the stratosphere and the normal distribution of ozone in the stratosphere. Without ozone depletion, most ozone is found in the lower stratosphere, near the Poles.

Why is ozone depletion greatest at polar regions? • During the long, dark winter, temperatures in the stratosphere fall to c. -80°C • Water, nitric acid and sulphuric acid form ice crystals • Ice crystals form to provide a suitable catalytic surface - (found on polar stratospheric clouds) • Spring sun in September activate dangerous forms of chlorine (ice melt) • Localised and seasonal increases in chlorine, thus making the ‘hole’ bigger and bigger in spring • Unreactive chlorine compounds into reactive chlorine radicals • The polar vortex has enhanced this process by isolating the cold polar air from the rest of the atmosphere in winter. • This hole gradually fills with warmer ozone rich air brought by the atmospheric circulation in the summer, however, as the hole is getting bigger, it is taking longer to fill • The ozone is also becoming thinner over the Arctic – which could possibly spread over North America affecting peoples health.

Are other gases to blame? • Oxides of Nitrogen have been identified, however amounts released within the troposphere do not reach the stratosphere due to reactions with other materials and it dissolving in rain • Aviation do release NOx – not enough for any significant change • Latest research has yet to be published on aviations long term impacts on ozone

The Montreal Protocol on Substances that Deplete the Ozone Layer " Perhaps the single most successful international agreement to date has been the Montreal Protocol. "-Kofi Annan, Former Secretary General of the United Nations

Timeline…events leading to the Montreal Protocol (1987) • 1985 the Vienna Convention established mechanisms for international co-operation in research into the ozone layer • 1985 marked the first discovery of the Antarctic ozone hole • Montreal Protocol on Substances that Deplete the Ozone Layer was negotiated and signed by 24 countries and by the European Economic Community in September 1987 • The Protocol called for the Parties to phase down the use of CFCs, halons and other man-made ODCs • one of the first international environmental agreements that includes trade sanctions to achieve the stated goals of a treaty

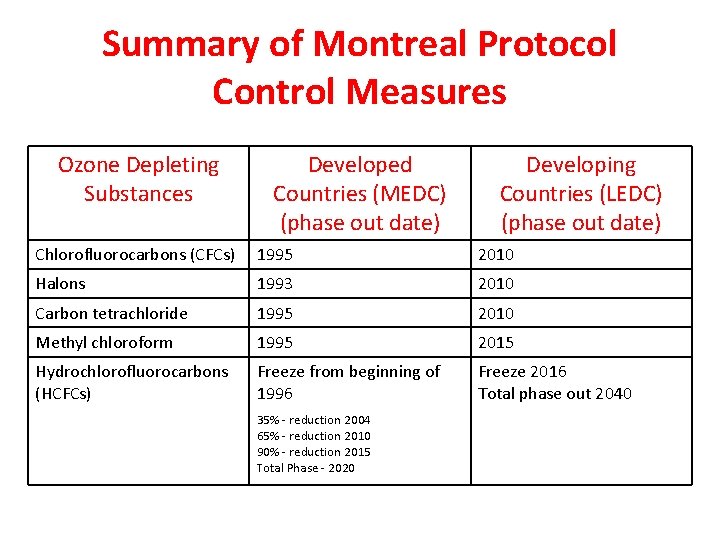
Summary of Montreal Protocol Control Measures Ozone Depleting Substances Developed Countries (MEDC) (phase out date) Developing Countries (LEDC) (phase out date) Chlorofluorocarbons (CFCs) 1995 2010 Halons 1993 2010 Carbon tetrachloride 1995 2010 Methyl chloroform 1995 2015 Hydrochlorofluorocarbons (HCFCs) Freeze from beginning of 1996 Freeze 2016 Total phase out 2040 35% - reduction 2004 65% - reduction 2010 90% - reduction 2015 Total Phase - 2020

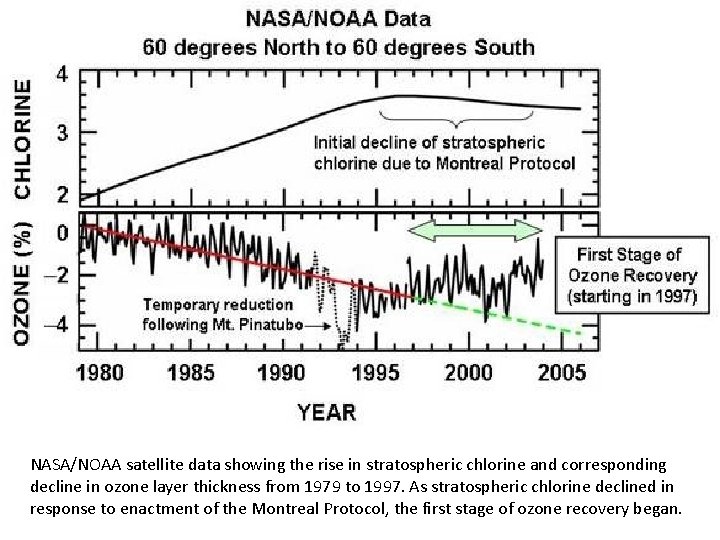
NASA/NOAA satellite data showing the rise in stratospheric chlorine and corresponding decline in ozone layer thickness from 1979 to 1997. As stratospheric chlorine declined in response to enactment of the Montreal Protocol, the first stage of ozone recovery began.

Questions… • Explain how humans activities have increased/caused ozone depletion (4 marks) • Outline the methods that have been used to reduce ozone depletion (8 marks) • Describe and explain why Antarctica is more susceptible to ozone depletion than equatorial regions (3 marks)

Summary of Ozone in the Stratosphere http: //www. esrl. noaa. gov/csd/assessments/ozo ne/2010/twentyquestions/