AOSC 620 The Ozone Hole R Dickerson Copyright






- Slides: 29

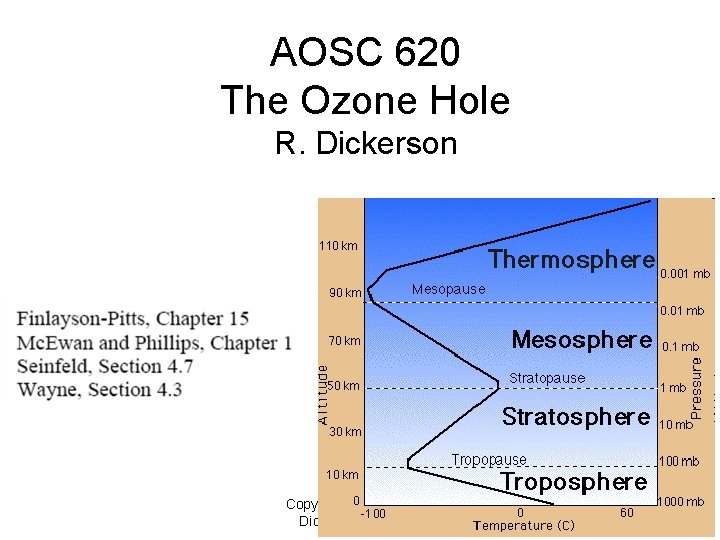
AOSC 620 The Ozone Hole R. Dickerson Copyright © 2010 R. R. Dickerson & Z. Q. Li 1

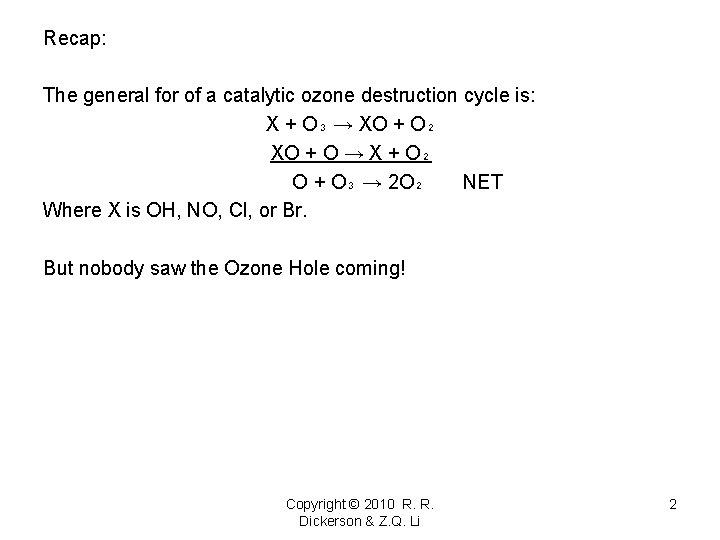
Recap: The general for of a catalytic ozone destruction cycle is: X + O₃ → XO + O₂ XO + O → X + O₂ O + O₃ → 2 O₂ NET Where X is OH, NO, Cl, or Br. But nobody saw the Ozone Hole coming! Copyright © 2010 R. R. Dickerson & Z. Q. Li 2

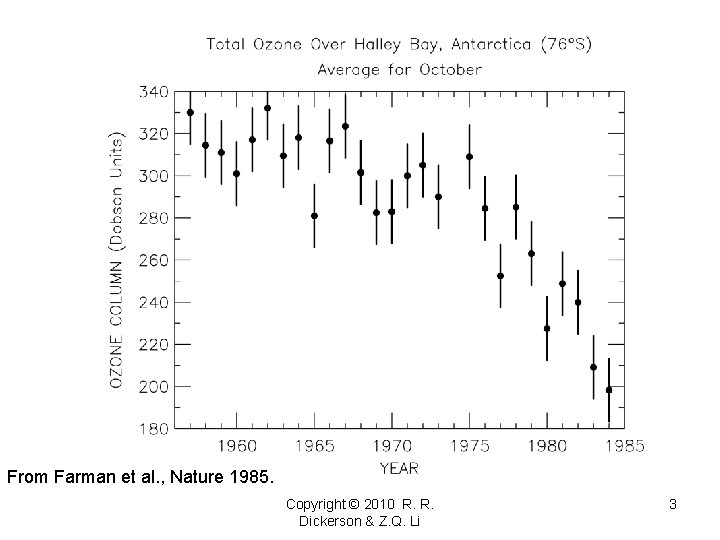
From Farman et al. , Nature 1985. Copyright © 2010 R. R. Dickerson & Z. Q. Li 3

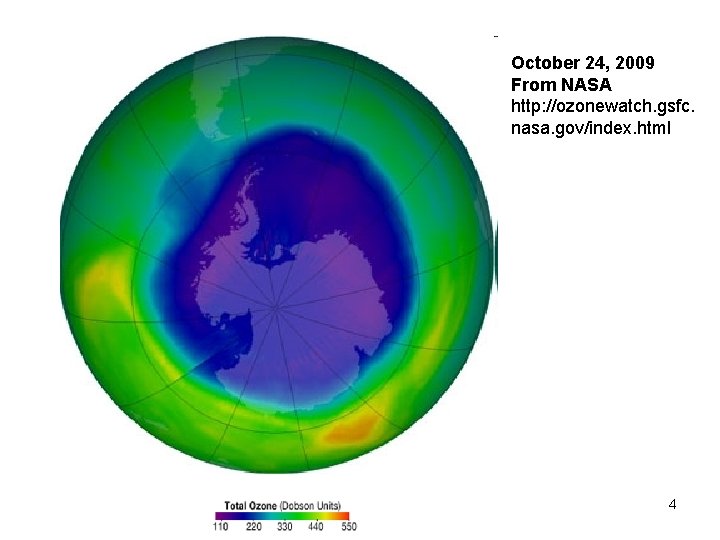
October 24, 2009 From NASA http: //ozonewatch. gsfc. nasa. gov/index. html Copyright © 2010 R. R. Dickerson & Z. Q. Li 4

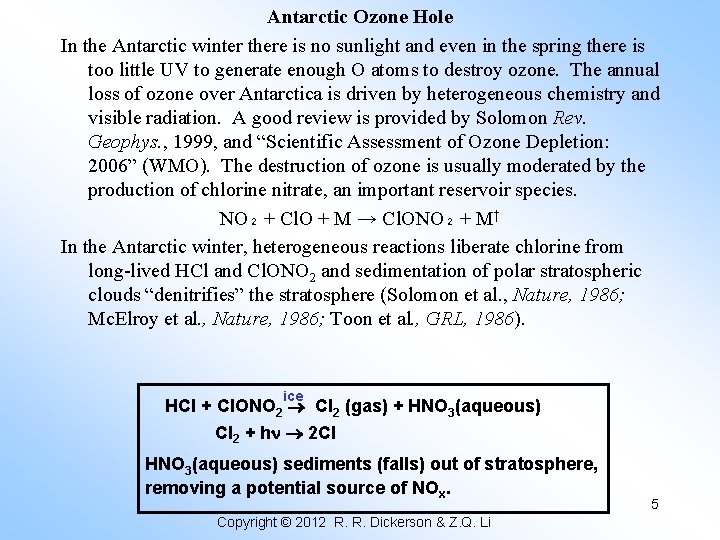
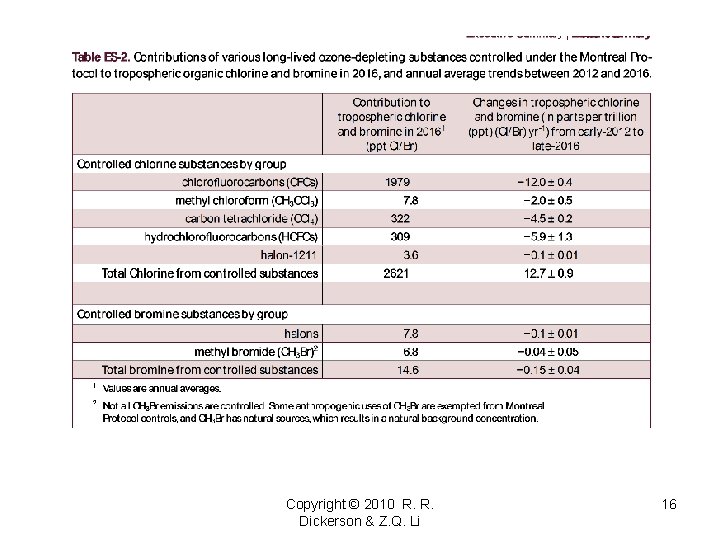
Antarctic Ozone Hole In the Antarctic winter there is no sunlight and even in the spring there is too little UV to generate enough O atoms to destroy ozone. The annual loss of ozone over Antarctica is driven by heterogeneous chemistry and visible radiation. A good review is provided by Solomon Rev. Geophys. , 1999, and “Scientific Assessment of Ozone Depletion: 2006” (WMO). The destruction of ozone is usually moderated by the production of chlorine nitrate, an important reservoir species. NO₂ + Cl. O + M → Cl. ONO₂ + M† In the Antarctic winter, heterogeneous reactions liberate chlorine from long-lived HCl and Cl. ONO 2 and sedimentation of polar stratospheric clouds “denitrifies” the stratosphere (Solomon et al. , Nature, 1986; Mc. Elroy et al. , Nature, 1986; Toon et al. , GRL, 1986). ice HCl + Cl. ONO 2 Cl 2 (gas) + HNO 3(aqueous) Cl 2 + h 2 Cl HNO 3(aqueous) sediments (falls) out of stratosphere, removing a potential source of NOx. Copyright © 2012 R. R. Dickerson & Z. Q. Li 5

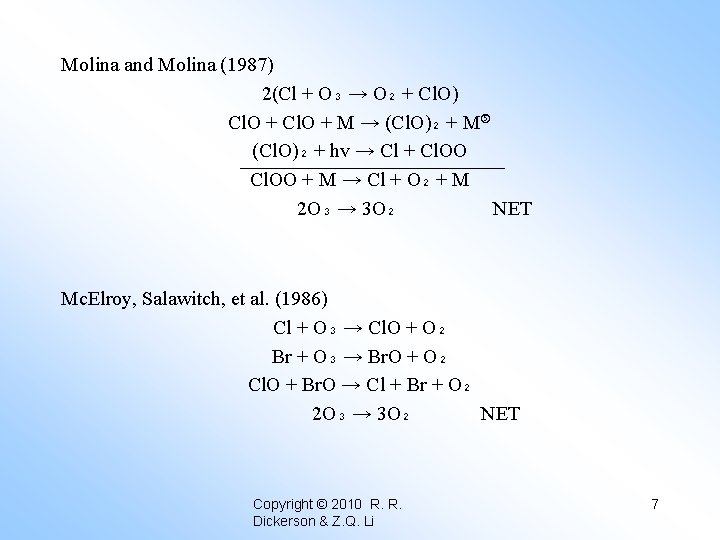
Molina and Molina (1987) 2(Cl + O₃ → O₂ + Cl. O) Cl. O + M → (Cl. O)₂ + M (Cl. O)₂ + hv → Cl + Cl. OO + M → Cl + O₂ + M 2 O₃ → 3 O₂ NET Two types of Polar Stratospheric Clouds (PSC’s) exist. Type I = HNO₃ ● 3 H₂O Nitric acid trihydrate, formed at T ≤ 195 K Type II = H₂O Water ice formed at T ≤ 190 K • They move NOy species from the vapor phase to the condensed phase as HNO₃. • They move chlorine from the reservoir species HCl and Cl. ONO₂ to Cl. Ox. Copyright © 2010 R. R. Dickerson & Z. Q. Li 6

Molina and Molina (1987) 2(Cl + O₃ → O₂ + Cl. O) Cl. O + M → (Cl. O)₂ + M (Cl. O)₂ + hv → Cl + Cl. OO + M → Cl + O₂ + M 2 O₃ → 3 O₂ NET Mc. Elroy, Salawitch, et al. (1986) Cl + O₃ → Cl. O + O₂ Br + O₃ → Br. O + O₂ Cl. O + Br. O → Cl + Br + O₂ 2 O₃ → 3 O₂ NET Copyright © 2010 R. R. Dickerson & Z. Q. Li 7

Airborne Antarctic Ozone Expedition: Punta Arenas, Chile, 1987 Anderson et al. , Science, 1991 Copyright © 2010 R. R. Dickerson & Z. Q. Li 8

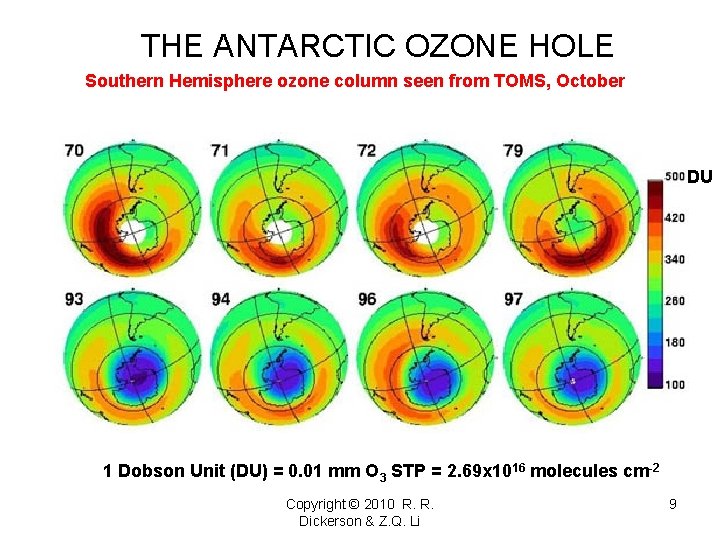
THE ANTARCTIC OZONE HOLE Southern Hemisphere ozone column seen from TOMS, October DU 1 Dobson Unit (DU) = 0. 01 mm O 3 STP = 2. 69 x 1016 molecules cm-2 Copyright © 2010 R. R. Dickerson & Z. Q. Li 9

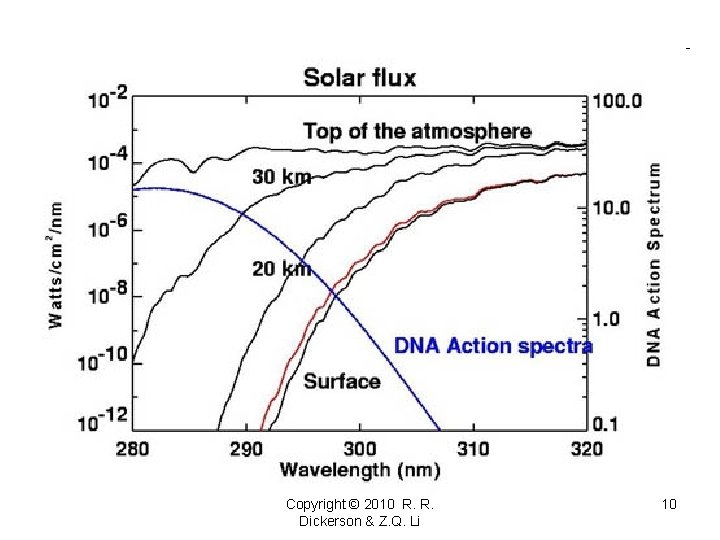
Copyright © 2010 R. R. Dickerson & Z. Q. Li 10

Polar Stratospheric Clouds (PSCs) Copyright © 2010 R. R. Dickerson & Z. Q. Li 11

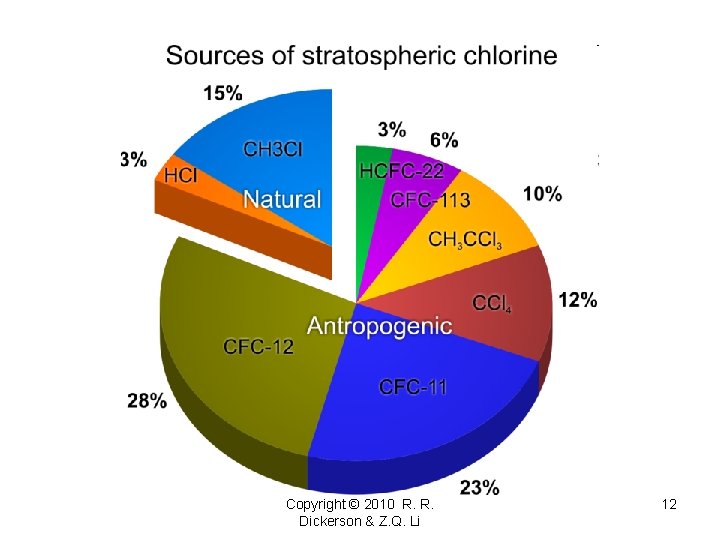
Copyright © 2010 R. R. Dickerson & Z. Q. Li 12

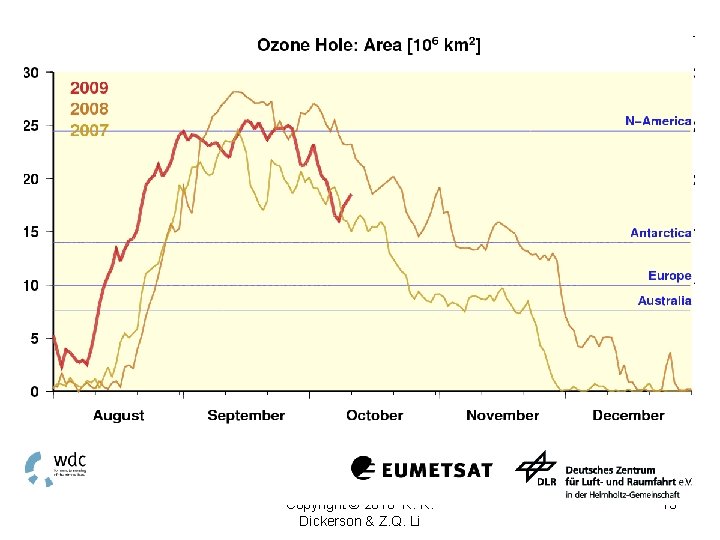
Copyright © 2010 R. R. Dickerson & Z. Q. Li 13

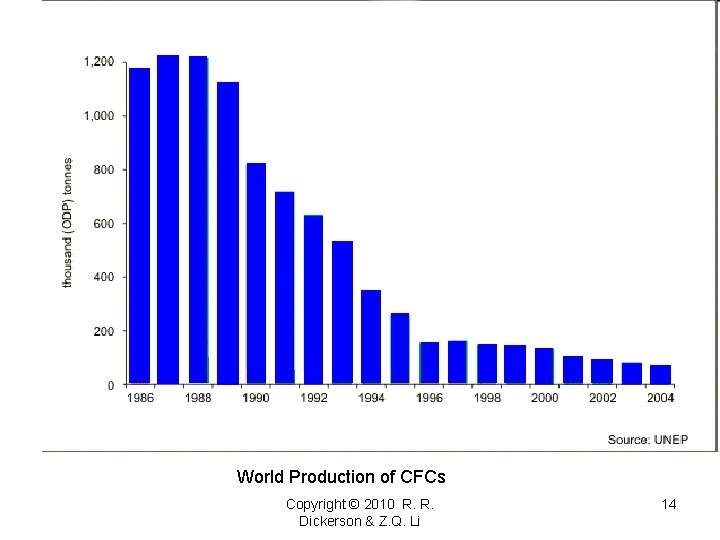
World Production of CFCs Copyright © 2010 R. R. Dickerson & Z. Q. Li 14

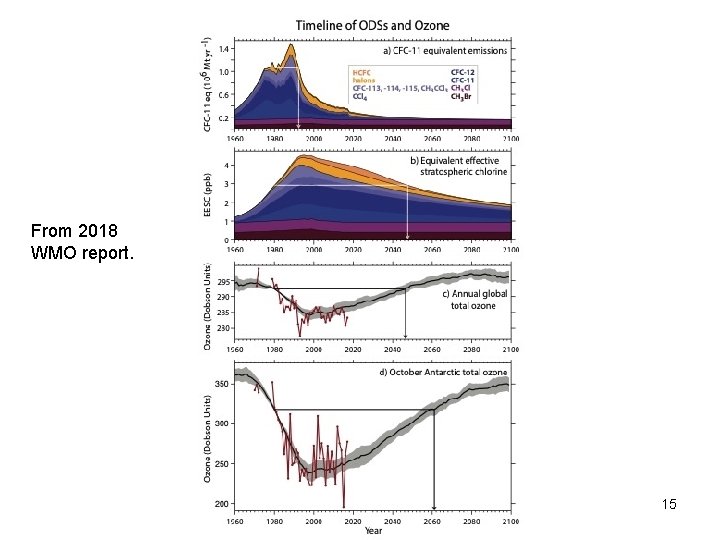
From 2018 WMO report. Copyright © 2010 R. R. Dickerson & Z. Q. Li 15

Copyright © 2010 R. R. Dickerson & Z. Q. Li 16

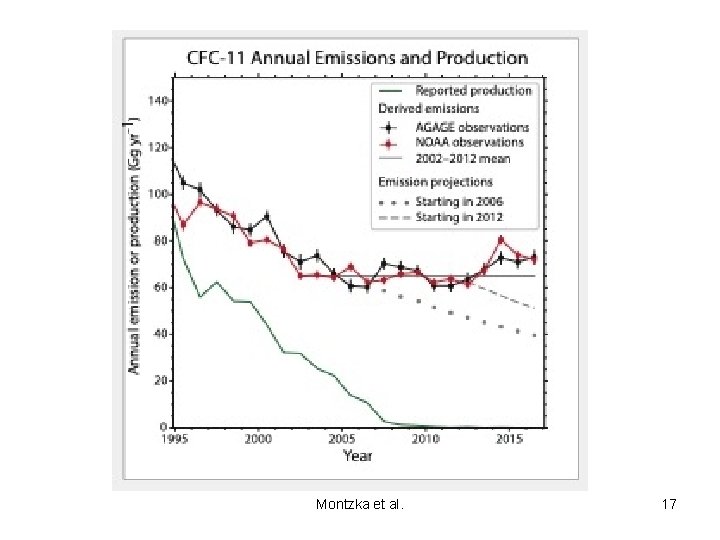
Montzka et al. 17

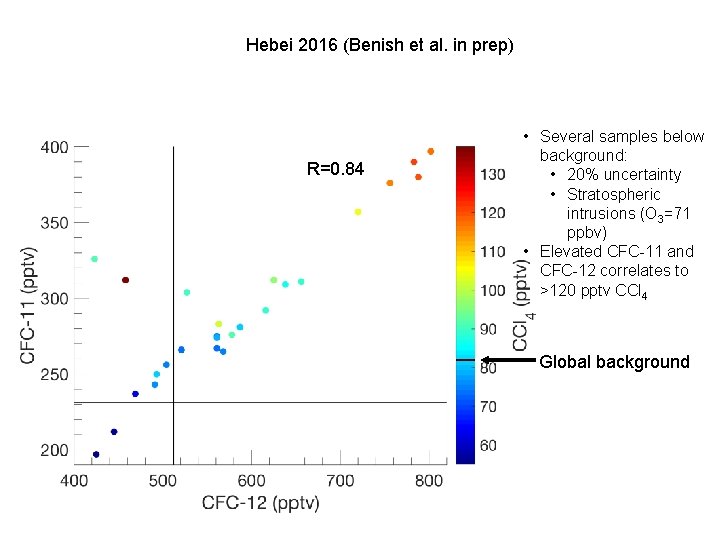
Hebei 2016 (Benish et al. in prep) R=0. 84 • Several samples below background: • 20% uncertainty • Stratospheric intrusions (O 3=71 ppbv) • Elevated CFC-11 and CFC-12 correlates to >120 pptv CCl 4 Global background

19

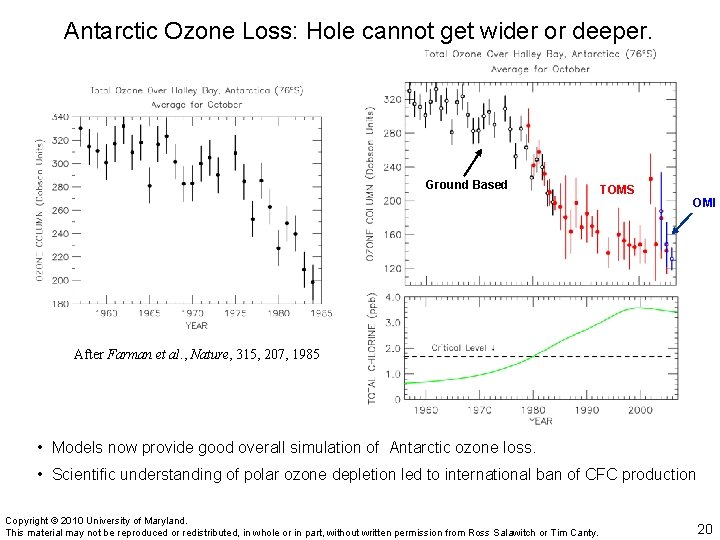
Antarctic Ozone Loss: Hole cannot get wider or deeper. Ground Based TOMS OMI After Farman et al. , Nature, 315, 207, 1985 • Models now provide good overall simulation of Antarctic ozone loss. • Scientific understanding of polar ozone depletion led to international ban of CFC production Copyright © 2010 University of Maryland. This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty. 20

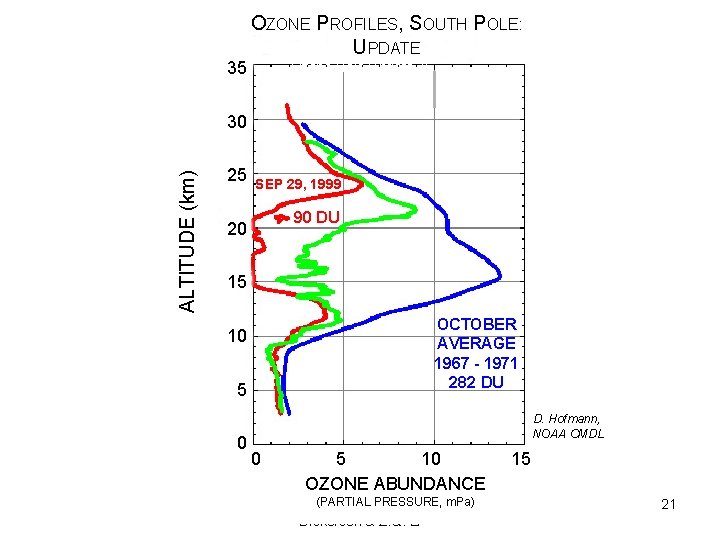
35 OZONE PROFILES, SOUTH POLE: UPDATE Ozone Hole Update, II ALTITUDE (km) 30 25 SEP 29, 1999 90 DU 20 15 OCTOBER AVERAGE 1967 - 1971 282 DU 10 5 0 D. Hofmann, NOAA CMDL 0 5 10 OZONE ABUNDANCE (PARTIAL PRESSURE, Copyright © 2010 R. R. m. Pa) Dickerson & Z. Q. Li 15 21

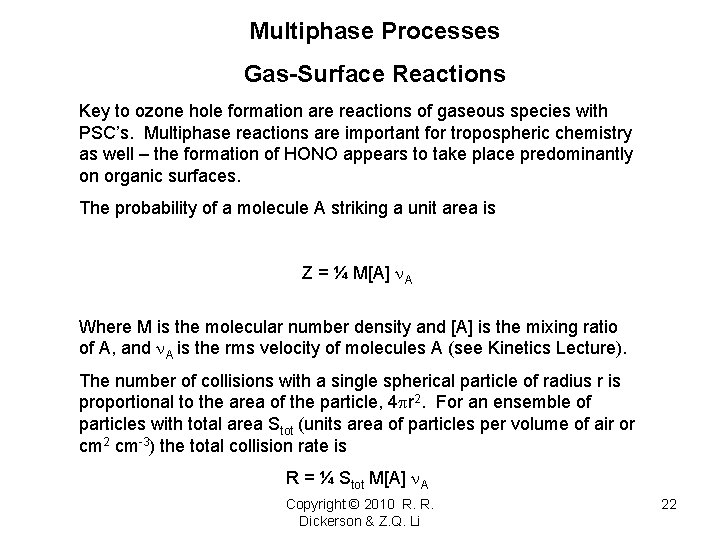
Multiphase Processes Gas-Surface Reactions Key to ozone hole formation are reactions of gaseous species with PSC’s. Multiphase reactions are important for tropospheric chemistry as well – the formation of HONO appears to take place predominantly on organic surfaces. The probability of a molecule A striking a unit area is Z = ¼ M[A] n. A Where M is the molecular number density and [A] is the mixing ratio of A, and n. A is the rms velocity of molecules A (see Kinetics Lecture). The number of collisions with a single spherical particle of radius r is proportional to the area of the particle, 4 pr 2. For an ensemble of particles with total area Stot (units area of particles per volume of air or cm 2 cm-3) the total collision rate is R = ¼ Stot M[A] n. A Copyright © 2010 R. R. Dickerson & Z. Q. Li 22

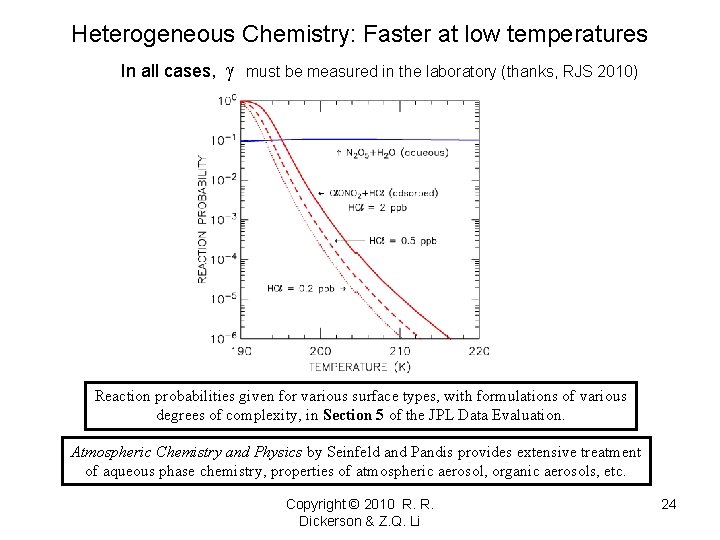
Accommodation Coefficients • Condensed phase has lower entropy than gas phase. • Accommodation coefficients (reaction probabilities) should be greater at lower temperatures. Copyright © 2010 R. R. Dickerson & Z. Q. Li 23

Heterogeneous Chemistry: Faster at low temperatures In all cases, must be measured in the laboratory (thanks, RJS 2010) Reaction probabilities given for various surface types, with formulations of various degrees of complexity, in Section 5 of the JPL Data Evaluation. Atmospheric Chemistry and Physics by Seinfeld and Pandis provides extensive treatment of aqueous phase chemistry, properties of atmospheric aerosol, organic aerosols, etc. Copyright © 2010 R. R. Dickerson & Z. Q. Li 24

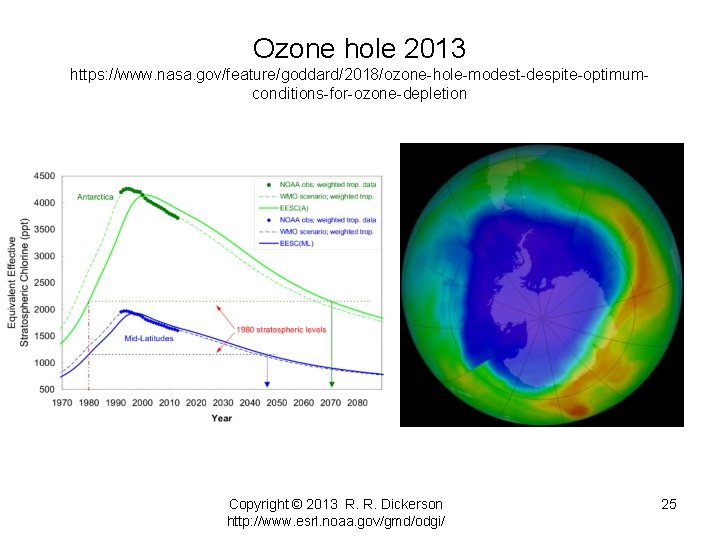
Ozone hole 2013 https: //www. nasa. gov/feature/goddard/2018/ozone-hole-modest-despite-optimumconditions-for-ozone-depletion Copyright © 2013 R. R. Dickerson http: //www. esrl. noaa. gov/gmd/odgi/ 25

Summary of Ozone Hole Formation • • Threat to DNA-based life forms. Not predicted by any models First observed by Farman et al. , (Nature 1985). Ozone destruction nearly complete. Halogen (Cl & Br) reactions are responsible. Polar stratospheric Clouds play a central role. Multiphase (heterogeneous) reactions denitrify stratosphere. • Reaction rates depend on accommodation coefficients, f(T). • Replacement of CFC’s appears to be healing ozone hole. Copyright © 2010 R. R. Dickerson & Z. Q. Li 26

Summary of Ozone Hole Formation • • Threat to DNA-based life forms. Not predicted by any models First observed by Farman et al. , (Nature 1985). Ozone destruction nearly complete. Halogen (Cl & Br) reactions are responsible. Polar stratospheric Clouds play a central role. Multiphase (heterogeneous) reactions denitrify stratosphere. • Reaction rates depend on accommodation coefficients, f(T). • Replacement of CFC’s should heal ozone hole. Copyright © 2010 R. R. Dickerson & Z. Q. Li 27

Latest findings (WMO/UNEP) • • • The Antarctic ozone hole is recovering, while continuing to occur every year. As a result of the Montreal Protocol much more severe ozone depletion in the polar regions has been avoided. Outside the polar regions, upper stratospheric ozone has increased by 1– 3% per decade since 2000. There has been an unexpected increase in global total emissions of CFC-11. Global CFC-11 emissions derived from measurements by two independent networks increased after 2012, slowing the steady decrease in atmospheric concentrations. The global concentration decline over 2014 to 2016 was only two thirds as fast as from 2002 to 2012. While the emissions of CFC-11 from eastern Asia have increased since 2012, the contribution of this region to the global emission rise is not well known. Copyright © 2010 R. R. Dickerson & Z. Q. Li 28

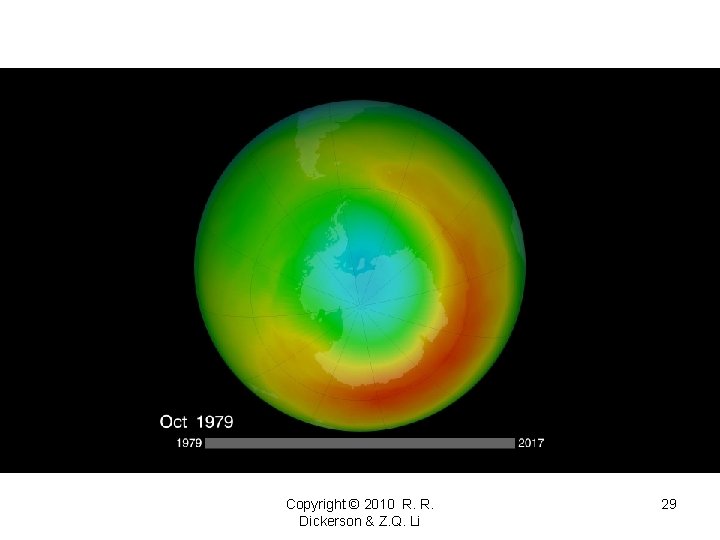
Copyright © 2010 R. R. Dickerson & Z. Q. Li 29
 Ozone hole myth
Ozone hole myth Evan dickerson
Evan dickerson John p. dickerson
John p. dickerson Sally dickerson
Sally dickerson Cmsc 320 github
Cmsc 320 github Krs 620
Krs 620 Aruba mesh ap
Aruba mesh ap Toefl 620
Toefl 620 Dd160 emc
Dd160 emc Here is where your presentation begins
Here is where your presentation begins Aircraft communications
Aircraft communications Measuring some 620 by 513 feet
Measuring some 620 by 513 feet Packing inst.
Packing inst. Buck colihue
Buck colihue Meam 620
Meam 620 Isa 620 using the work of an expert
Isa 620 using the work of an expert Dbhds incident reporting
Dbhds incident reporting Jms 620
Jms 620 Ozone klavye
Ozone klavye Ozone nasa
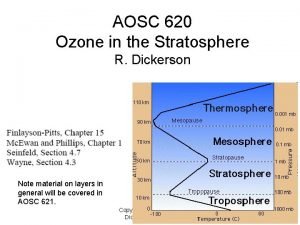
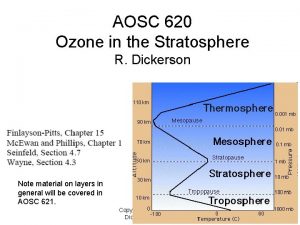
Ozone nasa How is total ozone distributed over the globe
How is total ozone distributed over the globe Lewis structures cannot
Lewis structures cannot Examples of non linear molecules
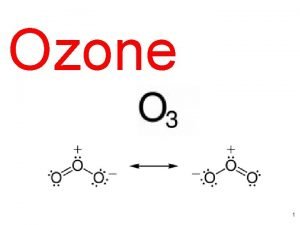
Examples of non linear molecules Ozone composition
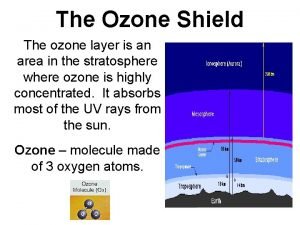
Ozone composition Ozone layer
Ozone layer Vray sun ozone
Vray sun ozone Ozone without borders
Ozone without borders Technic
Technic Protective ozone layer
Protective ozone layer Negative effects of ozone depletion
Negative effects of ozone depletion