3 Crystals What defines a crystal Atoms lattice



- Slides: 28

3. Crystals What defines a crystal? Atoms, lattice points, symmetry, space groups Diffraction B-factors Resolution Refinement Modeling!

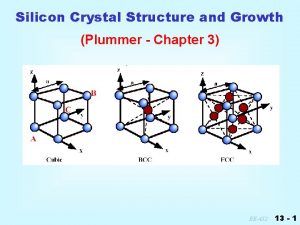
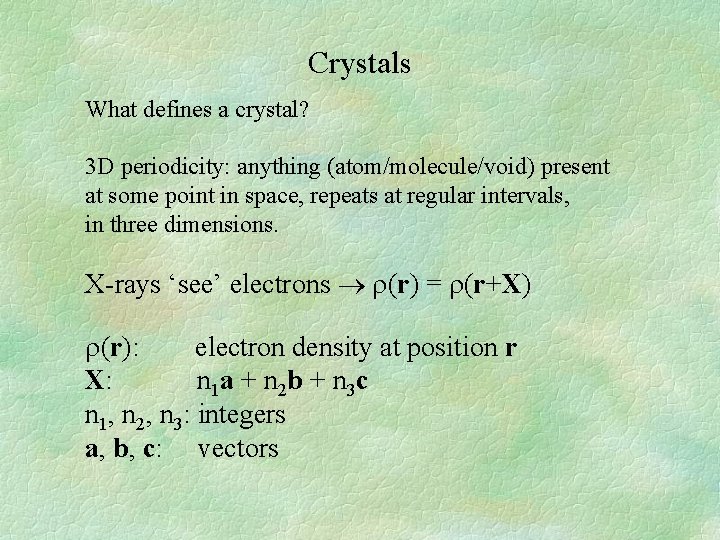
Crystals What defines a crystal? 3 D periodicity: anything (atom/molecule/void) present at some point in space, repeats at regular intervals, in three dimensions. X-rays ‘see’ electrons (r) = (r+X) (r): electron density at position r X: n 1 a + n 2 b + n 3 c n 1, n 2, n 3: integers a, b, c: vectors

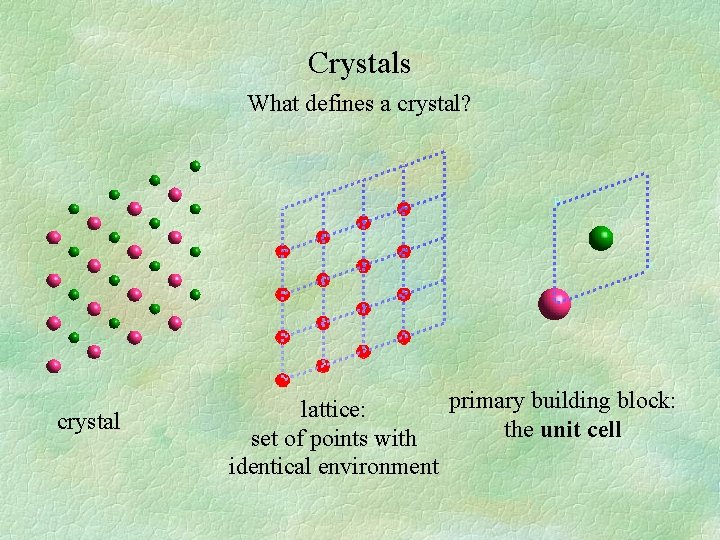
Crystals What defines a crystal? crystal primary building block: lattice: the unit cell set of points with identical environment

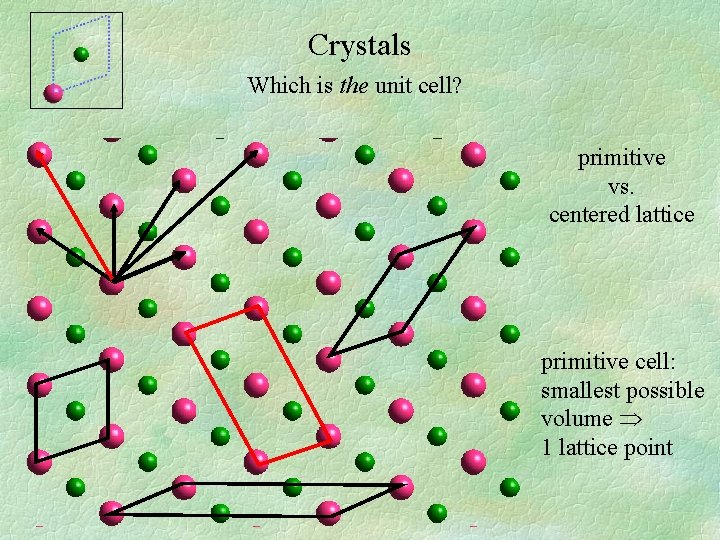
Crystals Which is the unit cell? primitive vs. centered lattice primitive cell: smallest possible volume 1 lattice point

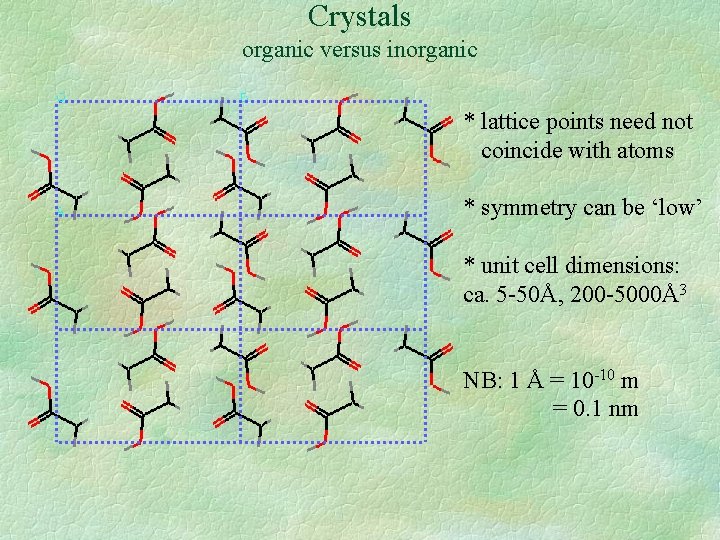
Crystals organic versus inorganic * lattice points need not coincide with atoms * symmetry can be ‘low’ * unit cell dimensions: ca. 5 -50Å, 200 -5000Å3 NB: 1 Å = 10 -10 m = 0. 1 nm

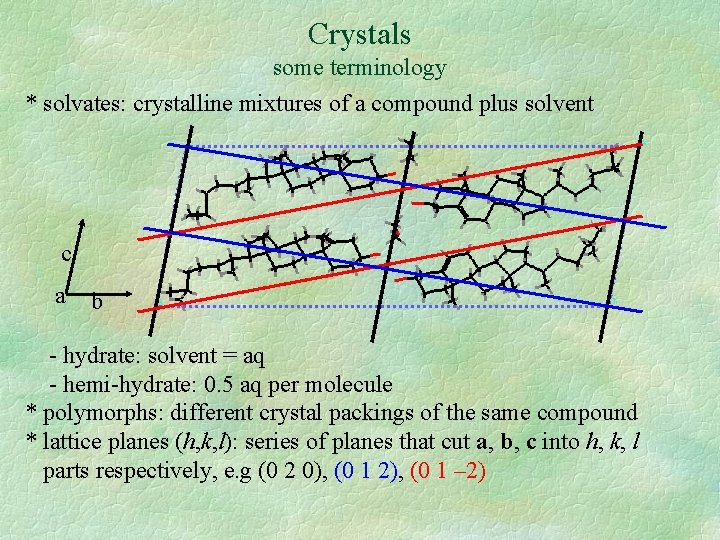
Crystals some terminology * solvates: crystalline mixtures of a compound plus solvent c a b - hydrate: solvent = aq - hemi-hydrate: 0. 5 aq per molecule * polymorphs: different crystal packings of the same compound * lattice planes (h, k, l): series of planes that cut a, b, c into h, k, l parts respectively, e. g (0 2 0), (0 1 2), (0 1 – 2)

Crystals coordinate systems Coordinates: positions of the atoms in the unit cell ‘carthesian: using Ångstrøms, and an ortho-normal system of axes. Practical e. g. when calculating distances. example: (5. 02, 9. 21, 3. 89) = the middle of the unit cell of estrone ‘fractional’: in fractions of the unit cell axes. Practical e. g. when calculating symmetry-related positions. examples: (½, ½, ½) = the middle of any unit cell (0. 1, 0. 2, 0. 3) and (-0. 2, 0. 1, 0. 3): symmetry related positions via axis of rotation along z-axis.

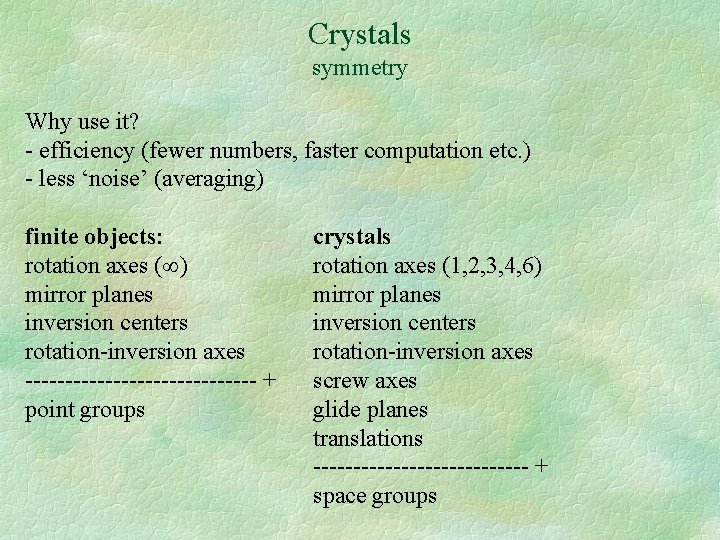
Crystals symmetry Why use it? - efficiency (fewer numbers, faster computation etc. ) - less ‘noise’ (averaging) finite objects: rotation axes ( ) mirror planes inversion centers rotation-inversion axes --------------- + point groups crystals rotation axes (1, 2, 3, 4, 6) mirror planes inversion centers rotation-inversion axes screw axes glide planes translations -------------- + space groups

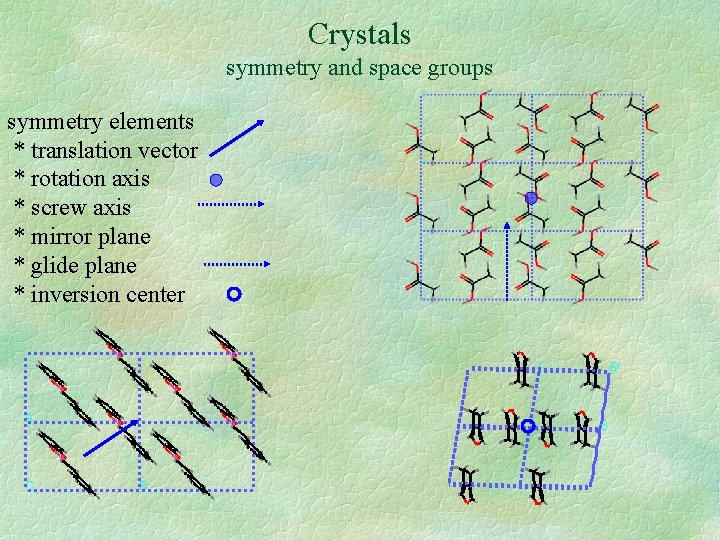
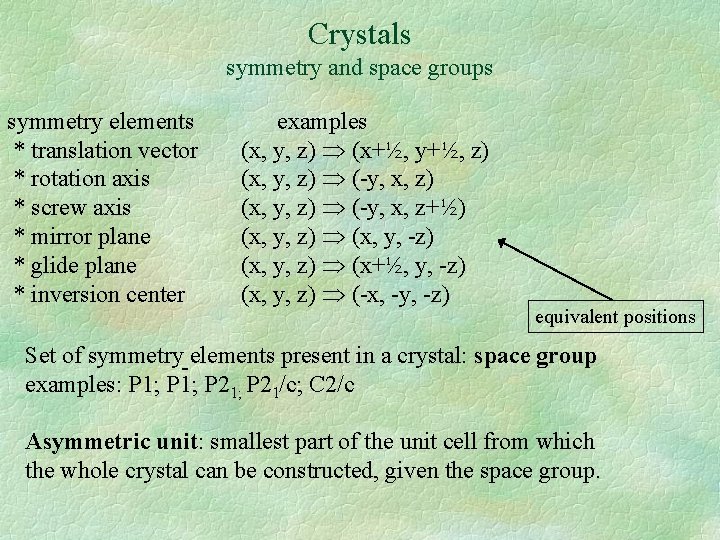
Crystals symmetry and space groups symmetry elements * translation vector * rotation axis * screw axis * mirror plane * glide plane * inversion center

Crystals symmetry and space groups symmetry elements * translation vector * rotation axis * screw axis * mirror plane * glide plane * inversion center examples (x, y, z) (x+½, y+½, z) (x, y, z) (-y, x, z+½) (x, y, z) (x, y, -z) (x, y, z) (x+½, y, -z) (x, y, z) (-x, -y, -z) equivalent positions Set of symmetry-elements present in a crystal: space group examples: P 1; P 21; P 21/c; C 2/c Asymmetric unit: smallest part of the unit cell from which the whole crystal can be constructed, given the space group.

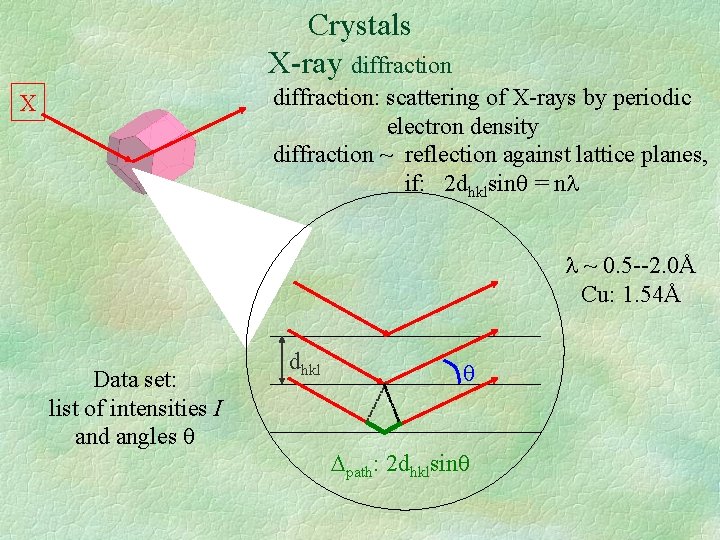
Crystals X-ray diffraction: scattering of X-rays by periodic electron density diffraction ~ reflection against lattice planes, if: 2 dhklsin = n X ~ 0. 5 --2. 0Å Cu: 1. 54Å Data set: list of intensities I and angles dhkl path: 2 dhklsin

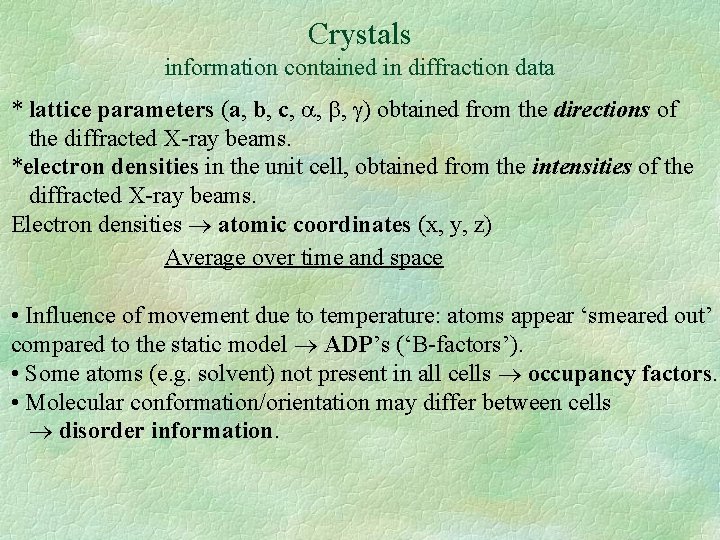
Crystals information contained in diffraction data * lattice parameters (a, b, c, , , ) obtained from the directions of the diffracted X-ray beams. *electron densities in the unit cell, obtained from the intensities of the diffracted X-ray beams. Electron densities atomic coordinates (x, y, z) Average over time and space • Influence of movement due to temperature: atoms appear ‘smeared out’ compared to the static model ADP’s (‘B-factors’). • Some atoms (e. g. solvent) not present in all cells occupancy factors. • Molecular conformation/orientation may differ between cells disorder information.

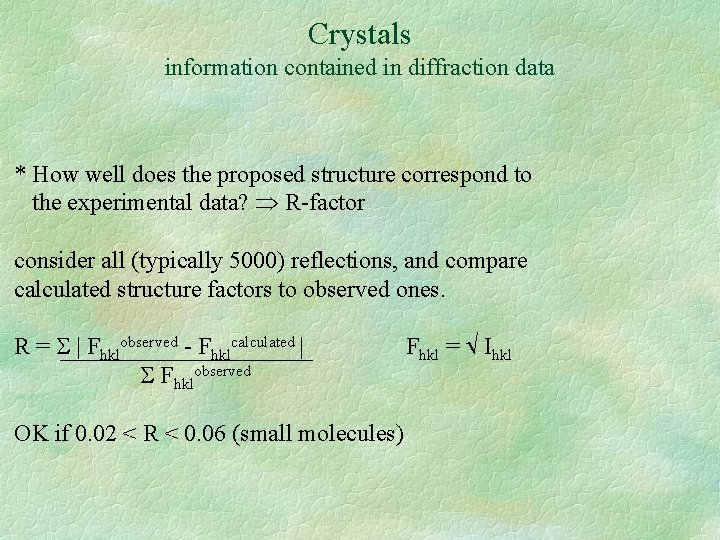
Crystals information contained in diffraction data * How well does the proposed structure correspond to the experimental data? R-factor consider all (typically 5000) reflections, and compare calculated structure factors to observed ones. R = | Fhklobserved - Fhklcalculated | Fhklobserved OK if 0. 02 < R < 0. 06 (small molecules) Fhkl = Ihkl

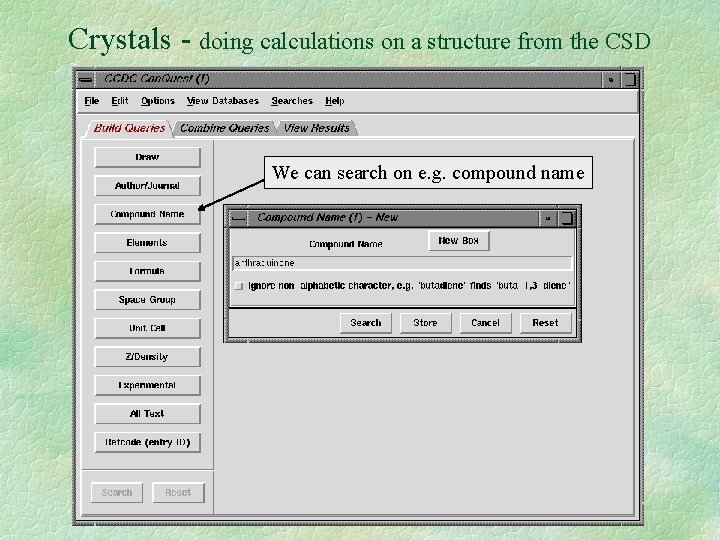
Crystals - doing calculations on a structure from the CSD We can search on e. g. compound name

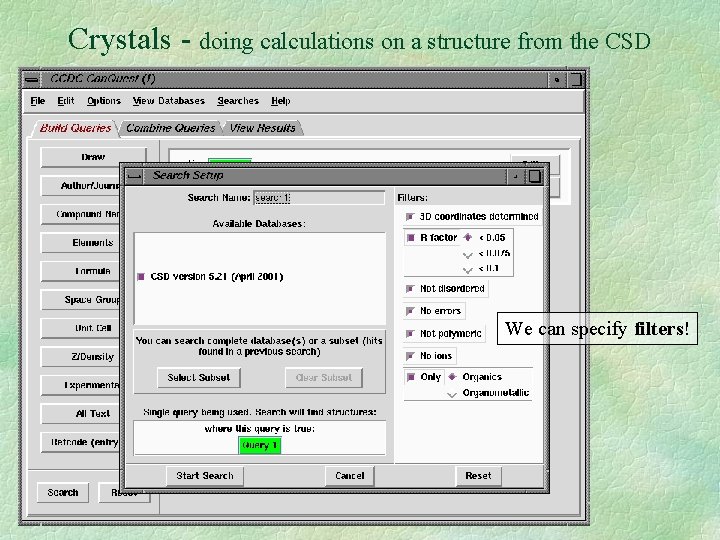
Crystals - doing calculations on a structure from the CSD We can specify filters!

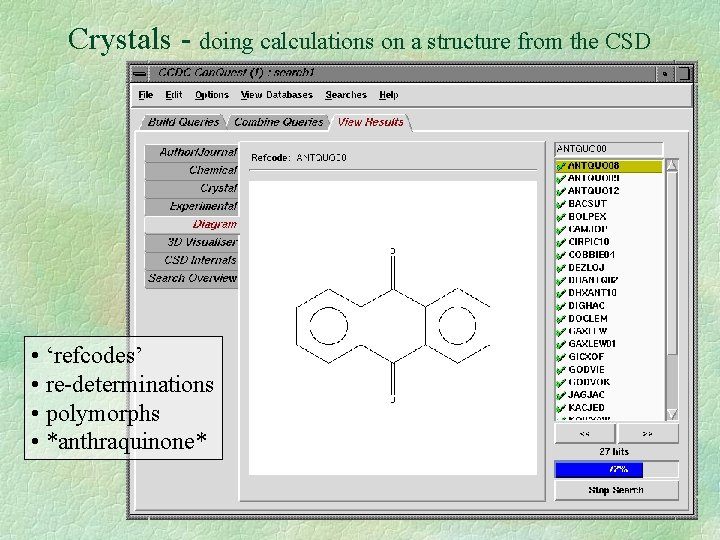
Crystals - doing calculations on a structure from the CSD • ‘refcodes’ • re-determinations • polymorphs • *anthraquinone*

Crystals - doing calculations on a structure from the CSD

Crystals - doing calculations on a structure from the CSD

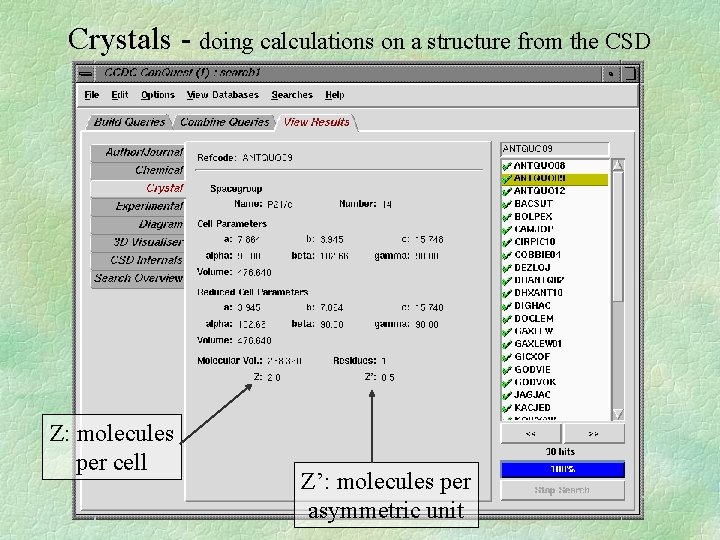
Crystals - doing calculations on a structure from the CSD Z: molecules per cell Z’: molecules per asymmetric unit

Crystals - doing calculations on a structure from the CSD

Crystals - doing calculations on a structure from the CSD

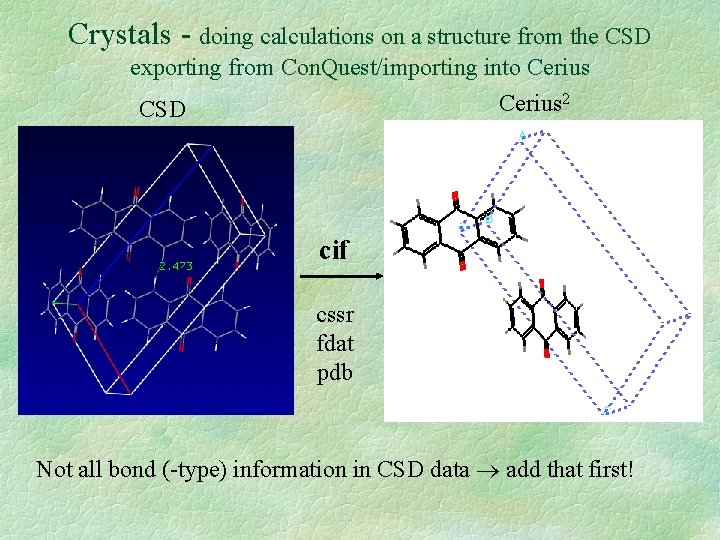
Crystals - doing calculations on a structure from the CSD exporting from Con. Quest/importing into Cerius 2 Cerius CSD cif cssr fdat pdb Not all bond (-type) information in CSD data add that first!

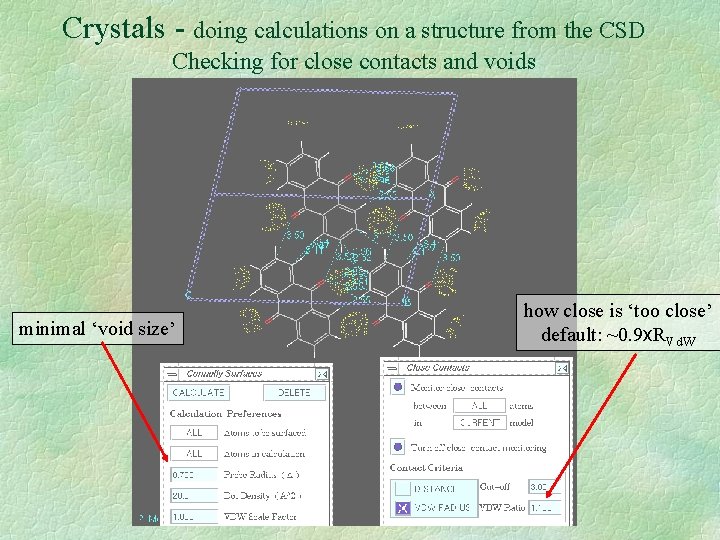
Crystals - doing calculations on a structure from the CSD Checking for close contacts and voids minimal ‘void size’ how close is ‘too close’ default: ~0. 9 x. RVd. W

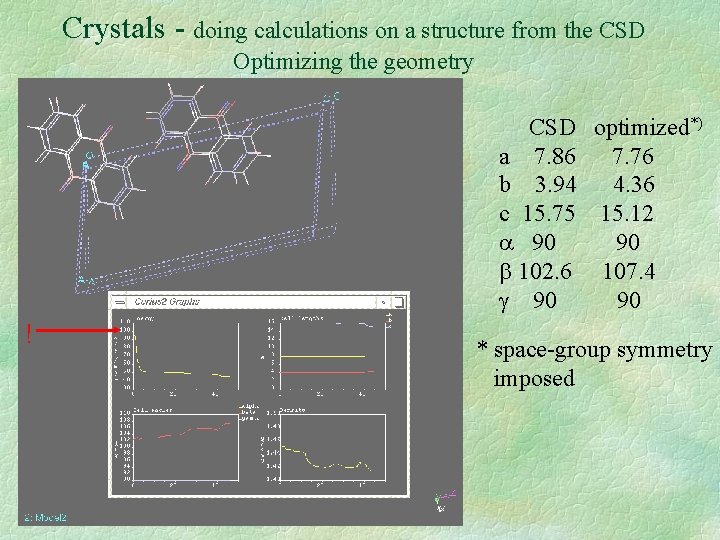
Crystals - doing calculations on a structure from the CSD Optimizing the geometry CSD optimized*) a 7. 86 7. 76 b 3. 94 4. 36 c 15. 75 15. 12 90 90 102. 6 107. 4 90 90 ! * space-group symmetry imposed

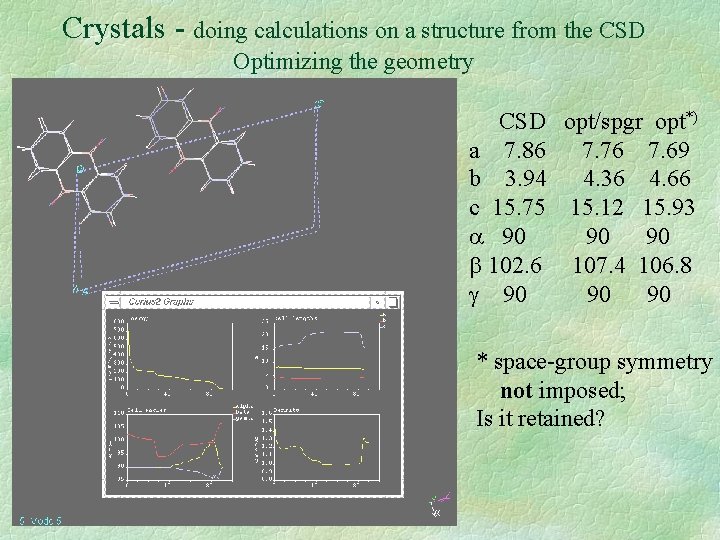
Crystals - doing calculations on a structure from the CSD Optimizing the geometry CSD opt/spgr opt*) a 7. 86 7. 76 7. 69 b 3. 94 4. 36 4. 66 c 15. 75 15. 12 15. 93 90 90 90 102. 6 107. 4 106. 8 90 90 90 * space-group symmetry not imposed; Is it retained?

Crystals - doing calculations on a structure from the CSD Optimizing the geometry Application of constraints during optimization: • space-group symmetry -- if assumed to be known • cell angles and/or axes -- e. g. from powder diffraction • positions of individual atoms -- e. g non-H, from diffraction • rigid bodies -- if molecule is rigid, or if it is too flexible. . .

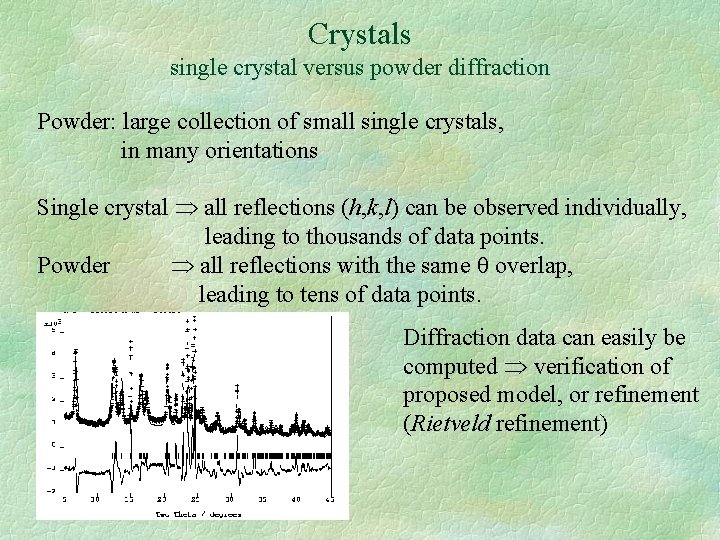
Crystals single crystal versus powder diffraction Powder: large collection of small single crystals, in many orientations Single crystal all reflections (h, k, l) can be observed individually, leading to thousands of data points. Powder all reflections with the same overlap, leading to tens of data points. Diffraction data can easily be computed verification of proposed model, or refinement (Rietveld refinement)

Next week…. Modeling crystals: how does it differ from small systems? Applications: predicting morphology predicting crystal packing
 Types of imperfections
Types of imperfections Silicon crystal lattice
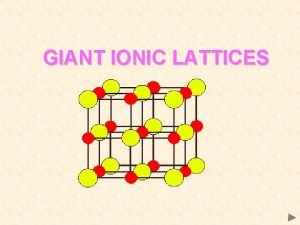
Silicon crystal lattice Giant ionic lattice
Giant ionic lattice Reciprocal lattice formula
Reciprocal lattice formula Poset matematika diskrit
Poset matematika diskrit At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Crystals
Crystals Barberios test
Barberios test Nacl structure
Nacl structure Rock candy hypothesis
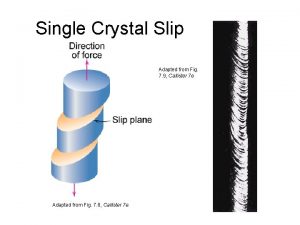
Rock candy hypothesis Slip plane
Slip plane Crystal binding energy
Crystal binding energy Uric acid crystals urine
Uric acid crystals urine Point defects
Point defects Copper ii sulphate crystals
Copper ii sulphate crystals Evaporation
Evaporation Uric
Uric Rock candy experiment conclusion
Rock candy experiment conclusion Are crystals pure substances
Are crystals pure substances Pauli crystals
Pauli crystals Cystine crystals in urine
Cystine crystals in urine Osazone crystals
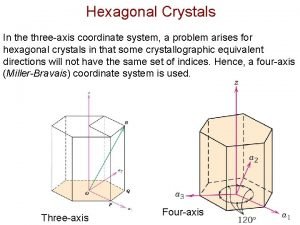
Osazone crystals Directions in hexagonal crystals
Directions in hexagonal crystals Largest crystals
Largest crystals Chapter 15 temperature heat and expansion
Chapter 15 temperature heat and expansion Cholesterol in urine
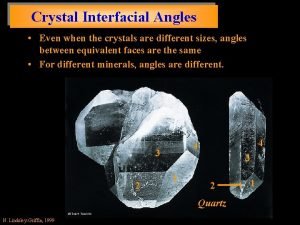
Cholesterol in urine Interfacial angles of crystals
Interfacial angles of crystals Mohamed akel
Mohamed akel Kato-katz and kato thick procedure
Kato-katz and kato thick procedure