Molecular Modeling of Crystal Structures molecules surfaces crystals
![Non-bonded interactions: Van der Waals repulsive: ~r-10 attractive: ~r-6 E=D 0[(r 0/r)12 -2(r 0/r)6] Non-bonded interactions: Van der Waals repulsive: ~r-10 attractive: ~r-6 E=D 0[(r 0/r)12 -2(r 0/r)6]](https://slidetodoc.com/presentation_image_h/1918bbd730a8c42c109446d3438534cf/image-12.jpg)





- Slides: 25

Molecular Modeling of Crystal Structures molecules surfaces crystals

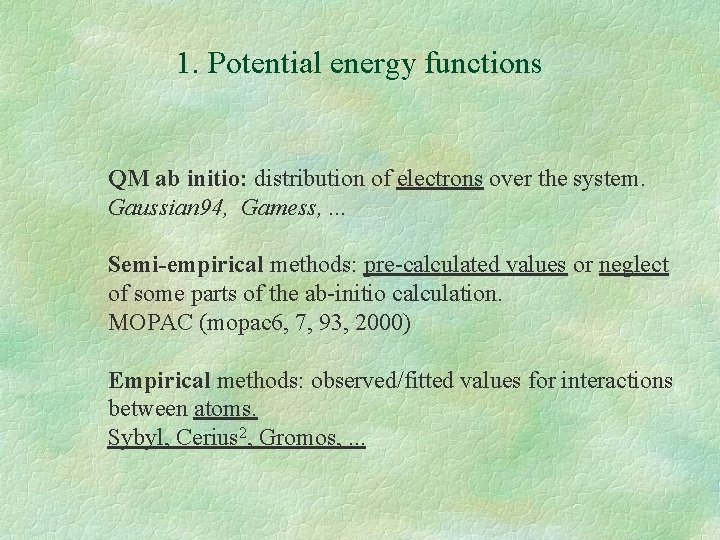
1. Potential energy functions QM ab initio: distribution of electrons over the system. Gaussian 94, Gamess, . . . Semi-empirical methods: pre-calculated values or neglect of some parts of the ab-initio calculation. MOPAC (mopac 6, 7, 93, 2000) Empirical methods: observed/fitted values for interactions between atoms. Sybyl, Cerius 2, Gromos, . . .

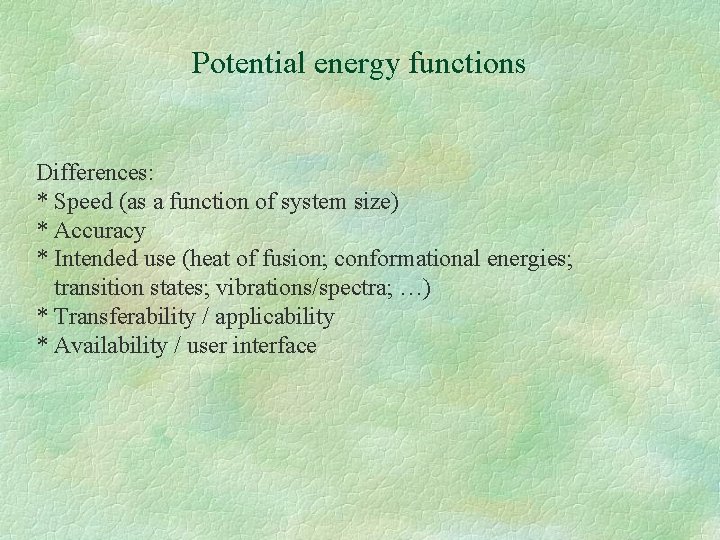
Potential energy functions Differences: * Speed (as a function of system size) * Accuracy * Intended use (heat of fusion; conformational energies; transition states; vibrations/spectra; …) * Transferability / applicability * Availability / user interface

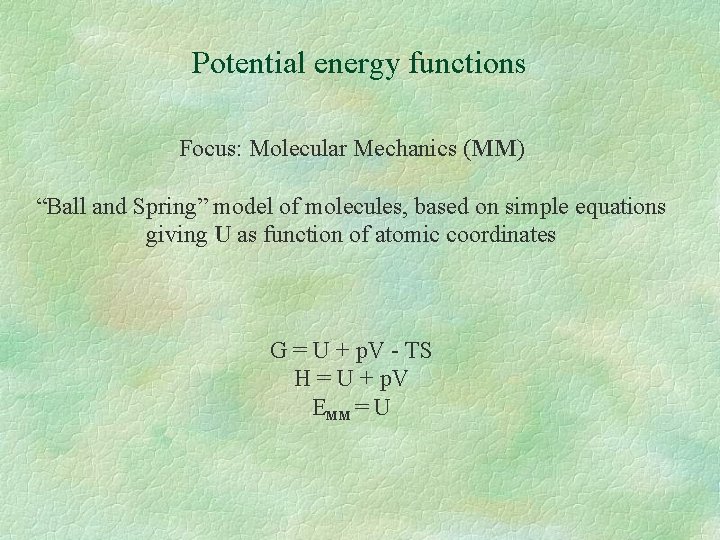
Potential energy functions Focus: Molecular Mechanics (MM) “Ball and Spring” model of molecules, based on simple equations giving U as function of atomic coordinates G = U + p. V - TS H = U + p. V EMM = U

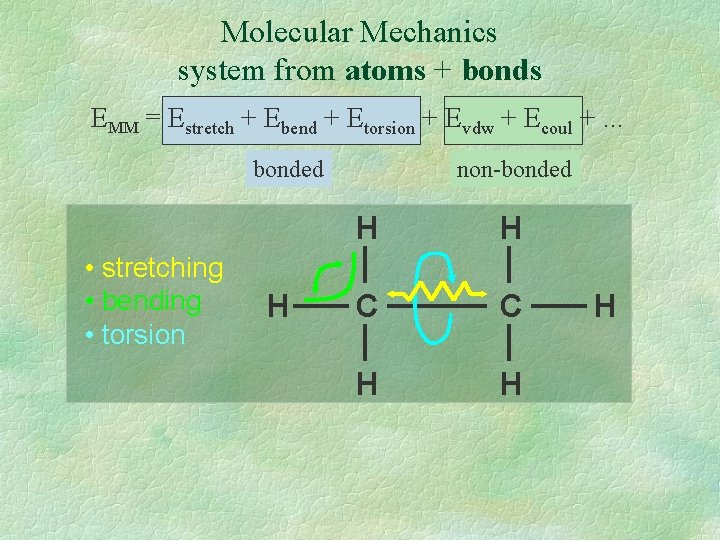
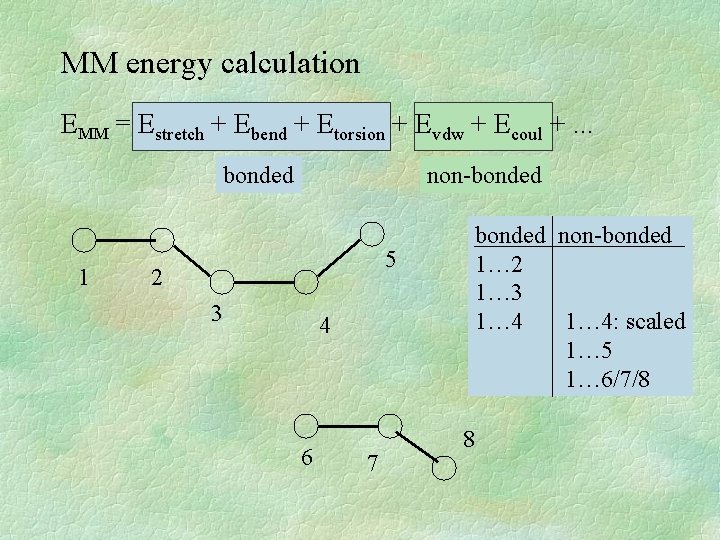
Molecular Mechanics system from atoms + bonds EMM = Estretch + Ebend + Etorsion + Evdw + Ecoul +. . . bonded • stretching • bending • torsion H non-bonded H H C C H H H

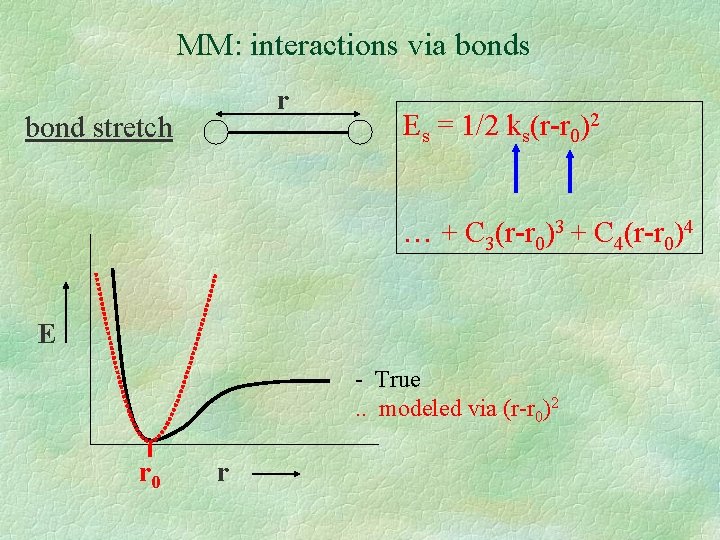
MM: interactions via bonds r bond stretch Es = 1/2 ks(r-r 0)2 … + C 3(r-r 0)3 + C 4(r-r 0)4 E - True. . modeled via (r-r 0)2 r 0 r

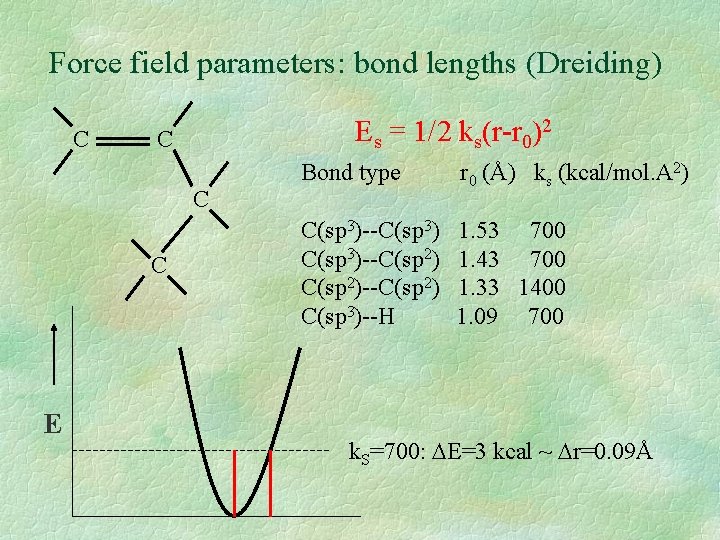
Force field parameters: bond lengths (Dreiding) C Es = 1/2 ks(r-r 0)2 C C C E Bond type r 0 (Å) ks (kcal/mol. A 2) C(sp 3)--C(sp 3)--C(sp 2)--C(sp 2) C(sp 3)--H 1. 53 700 1. 43 700 1. 33 1400 1. 09 700 k. S=700: E=3 kcal ~ r=0. 09Å

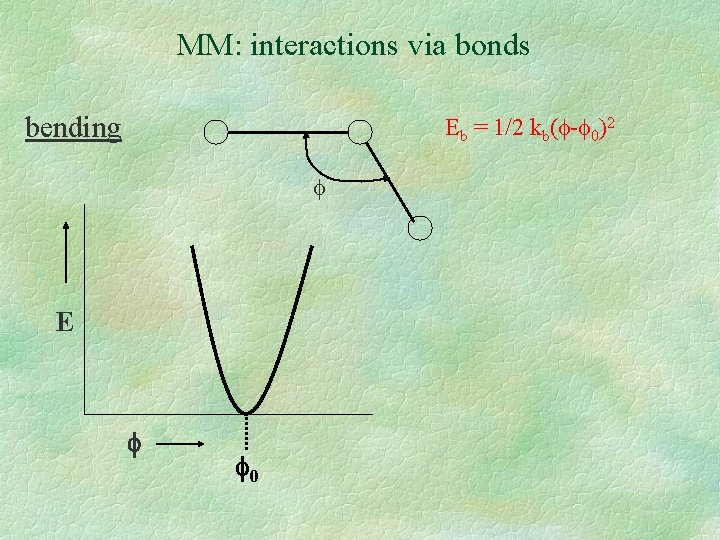
MM: interactions via bonds bending Eb = 1/2 kb( - 0)2 E 0

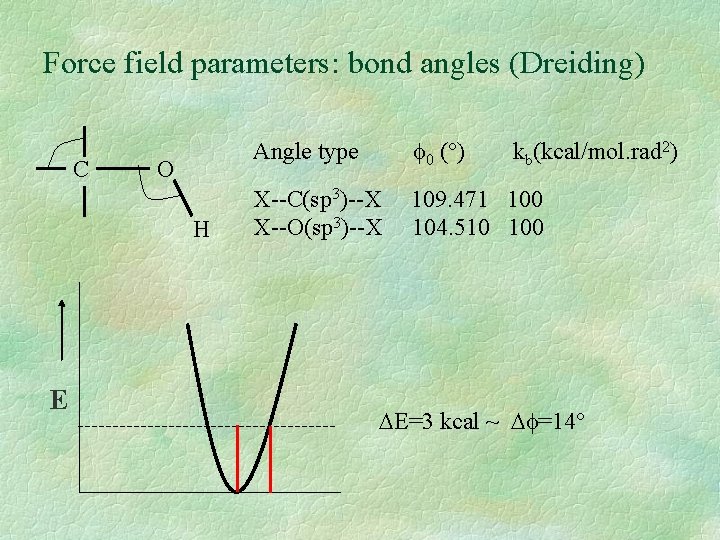
Force field parameters: bond angles (Dreiding) C O H E Angle type 0 (°) X--C(sp 3)--X X--O(sp 3)--X 109. 471 100 104. 510 100 kb(kcal/mol. rad 2) E=3 kcal ~ =14°

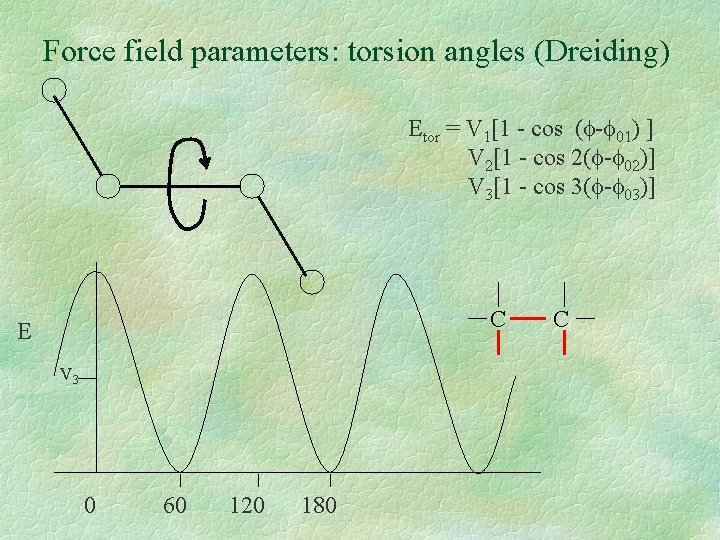
Force field parameters: torsion angles (Dreiding) Etor = V 1[1 - cos ( - 01) ] V 2[1 - cos 2( - 02)] V 3[1 - cos 3( - 03)] C E V 3 0 60 120 180 C

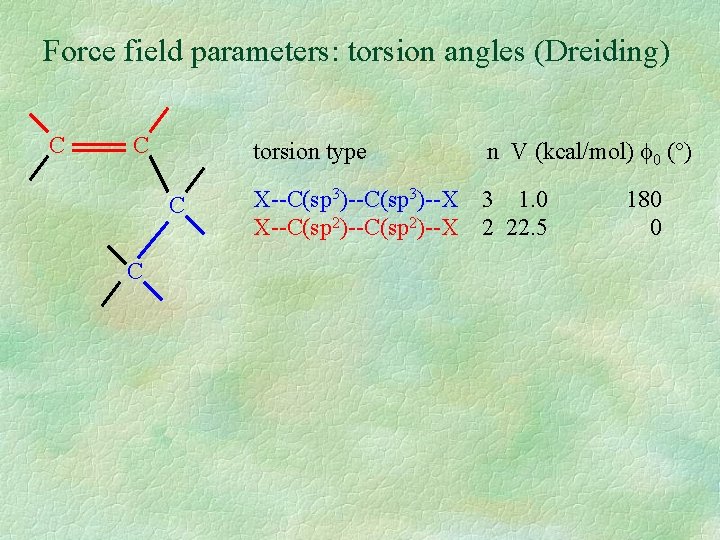
Force field parameters: torsion angles (Dreiding) C C torsion type n V (kcal/mol) 0 (°) X--C(sp 3)--X X--C(sp 2)--X 3 1. 0 2 22. 5 180 0
![Nonbonded interactions Van der Waals repulsive r10 attractive r6 ED 0r 0r12 2r 0r6 Non-bonded interactions: Van der Waals repulsive: ~r-10 attractive: ~r-6 E=D 0[(r 0/r)12 -2(r 0/r)6]](https://slidetodoc.com/presentation_image_h/1918bbd730a8c42c109446d3438534cf/image-12.jpg)
Non-bonded interactions: Van der Waals repulsive: ~r-10 attractive: ~r-6 E=D 0[(r 0/r)12 -2(r 0/r)6] (Lennard-Jones) E=D 0{exp[a(r 0/r)]-b(r 0/r)6} (Buckingham; “exp-6”)

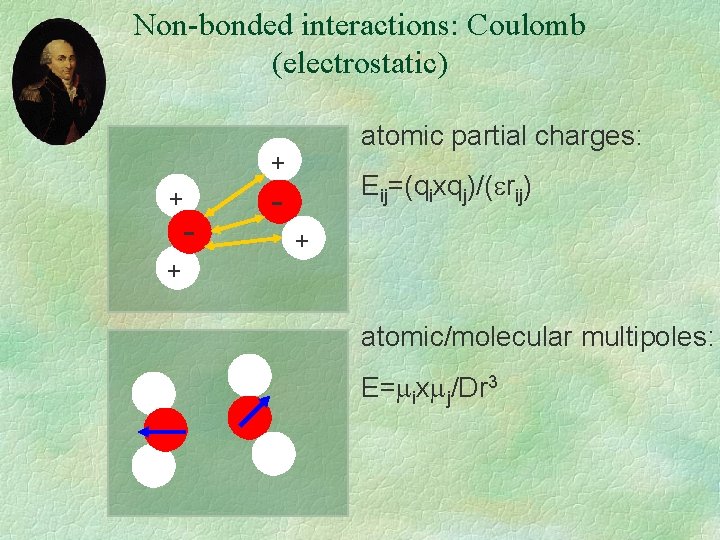
Non-bonded interactions: Coulomb (electrostatic) atomic partial charges: + + - Eij=(qixqj)/( rij) + + atomic/molecular multipoles: E= ix j/Dr 3

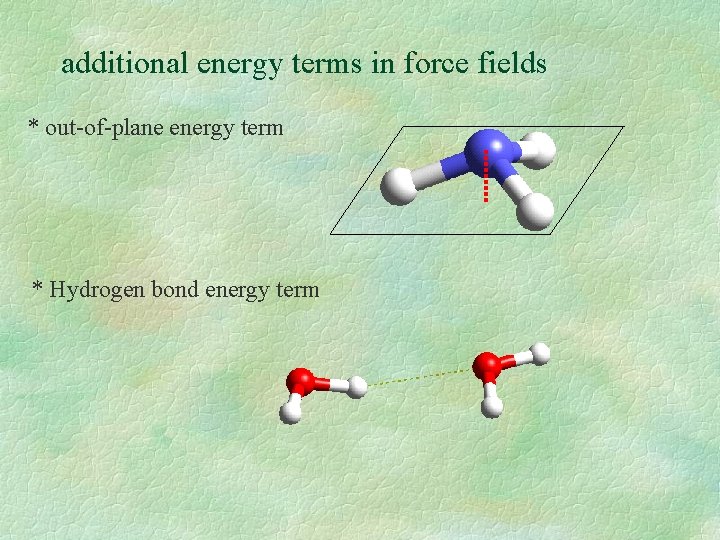
additional energy terms in force fields * out-of-plane energy term * Hydrogen bond energy term

MM energy calculation EMM = Estretch + Ebend + Etorsion + Evdw + Ecoul +. . . bonded 1 non-bonded 5 2 3 4 6 7 bonded non-bonded 1… 2 1… 3 1… 4: scaled 1… 5 1… 6/7/8 8

Some available force fields FF software Gromos Charmm; Quanta Amber Tripos Sybyl Dreiding Cerius Compass Cerius CVFF Cerius Glass 2. 01 Cerius focus bio bio general ionic

Force field parameters: where do they come from? 1. Mimic physical properties of individual elements or atom types, producing a “physical” force field. Properties can be taken from experimental data, or ab-initio calculations. Examples: Dreiding, Compass. + outcome will be ‘reasonable’, predictable; extension to new systems relatively straightforward. - performance not very good.

Force field parameters: where do they come from? 2. Optimize all parameters with respect to a set of test data, producing a “consistent” force field. Test set can be chosen to represent the system under investigation. Examples: CFF, CVFF. + outcome often good for a particular type of systems, or a particular property (e. g. IR spectrum). - extension to new systems can be difficult; no direct link to ‘physical reality’

Force field parameters: where do they come from? 3. Apply common sense and look at what the neighbors do. Examples: Gromos. + does not waste time on FF parameterization; resonable results. -?

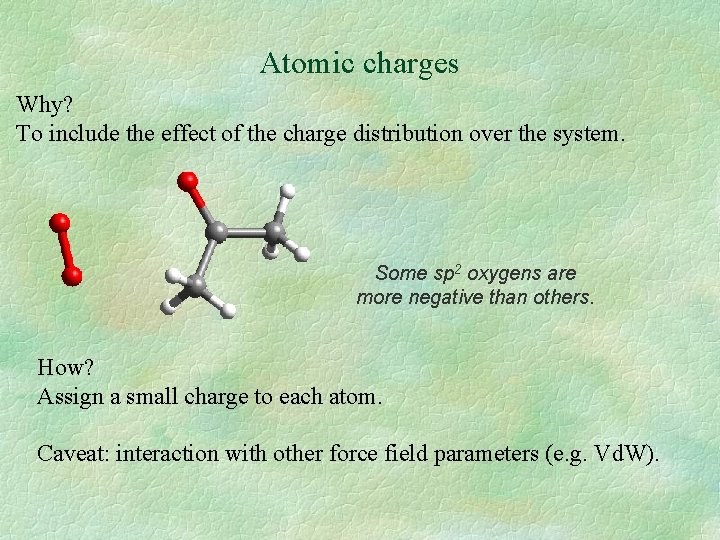
Atomic charges Why? To include the effect of the charge distribution over the system. Some sp 2 oxygens are more negative than others. How? Assign a small charge to each atom. Caveat: interaction with other force field parameters (e. g. Vd. W).

Atomic charges What is the atomic charge? * Based on atomic electronegativity, optimized for a given FF. example: Gasteiger charges. • Based on atomic electronegativity and the resulting electrical field. example: Charge Equilibrium charges (QEq). * Based on the electronic distribution calculated by QM. example: Mulliken charges. * Based on the electrostatic potential near the molecule, calculated by a non-empirical method (or determined experimentally). examples: Chelp, Chelp. G, RESP.

Atomic charges Properties and features of different charge schemes: * Depends on molecular conformation? * Easy (=quick) to calculate? * Performance in combination with force field? Known-to-be-good combinations: Tripos -- Gasteiger Dreiding -- ESP Compass -- Compass

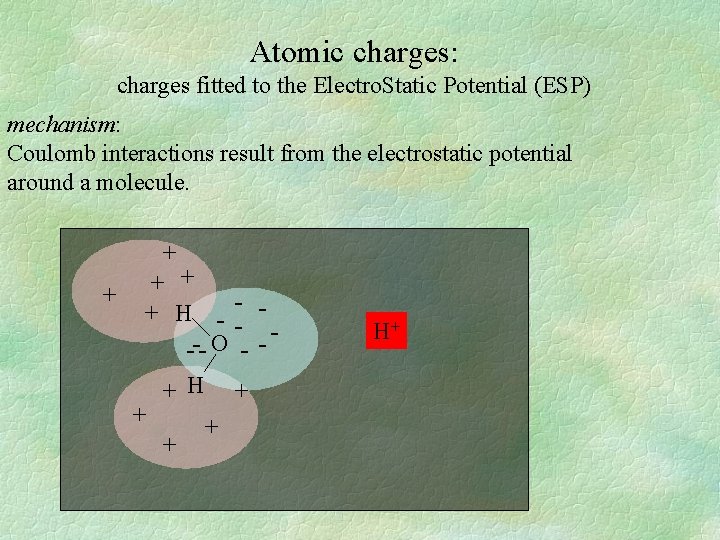
Atomic charges: charges fitted to the Electro. Static Potential (ESP) mechanism: Coulomb interactions result from the electrostatic potential around a molecule. + + + H - -- O -- + H + + H+

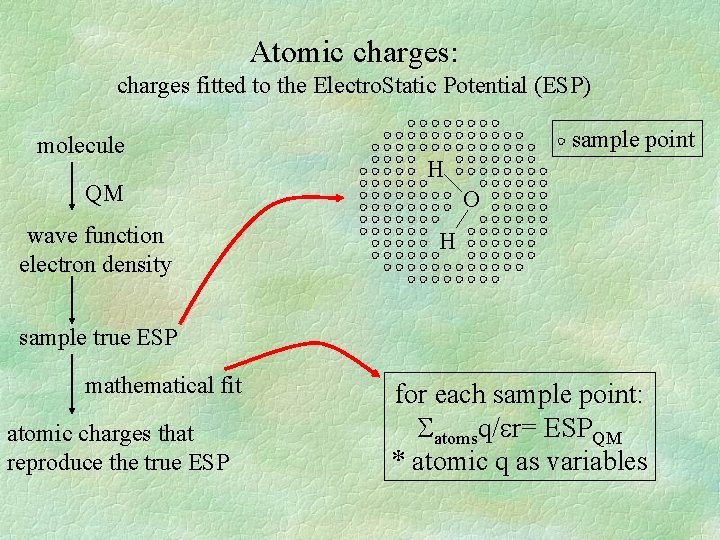
Atomic charges: charges fitted to the Electro. Static Potential (ESP) molecule QM wave function electron density sample point H O H sample true ESP mathematical fit atomic charges that reproduce the true ESP for each sample point: atomsq/ r= ESPQM * atomic q as variables

Atomic charges: charges fitted to the Electro. Static Potential (ESP) Properties and features of different fitting schemes: * Number of sample points. * Position of sample points. * Additional restraints (e. g. all q. H in CH 3 equal). * Fitting to multiple conformations. Known-to-be-good fitting schemes: Chelp. G RESP