Lattice Energies AP Material Lattice Energy l Lattice






- Slides: 11

Lattice Energies AP Material

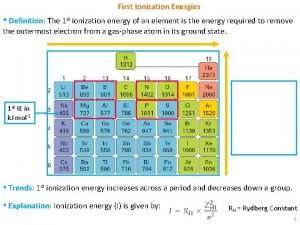
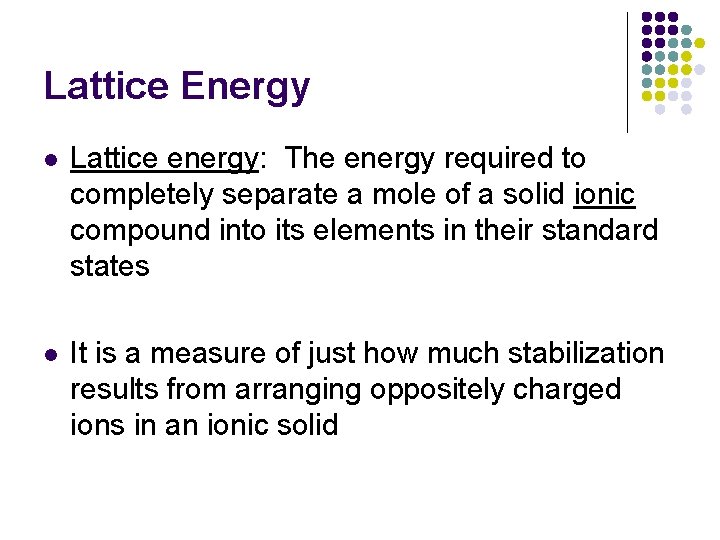
Lattice Energy l Lattice energy: The energy required to completely separate a mole of a solid ionic compound into its elements in their standard states l It is a measure of just how much stabilization results from arranging oppositely charged ions in an ionic solid

l The principal reason that ionic compounds are stable is the attraction between oppositely charged ions l This attraction draws the ions together, releasing energy and causing ions to form a solid array, or lattice l http: //www. youtube. com/watch? v=VBRe. Ojo 3 ri 8&feature=related

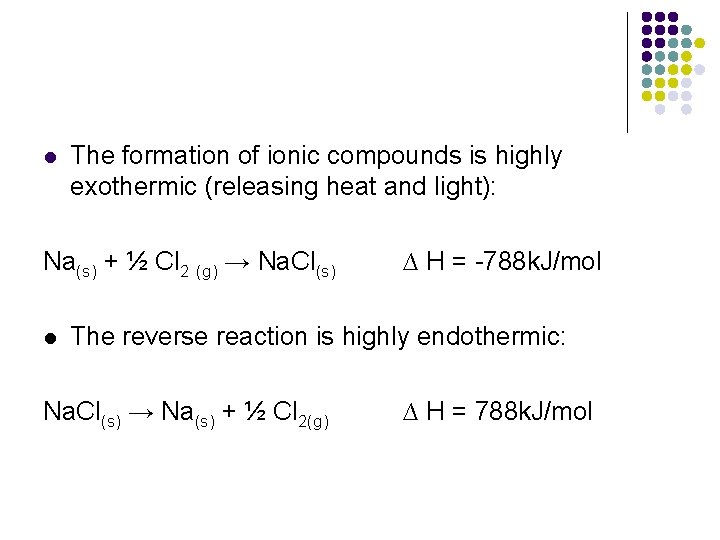
l The formation of ionic compounds is highly exothermic (releasing heat and light): Na(s) + ½ Cl 2 (g) → Na. Cl(s) l ∆ H = -788 k. J/mol The reverse reaction is highly endothermic: Na. Cl(s) → Na(s) + ½ Cl 2(g) ∆ H = 788 k. J/mol

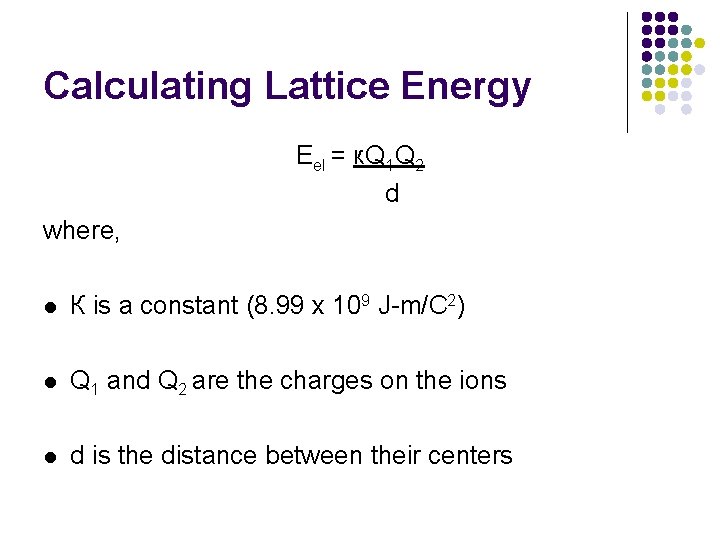
Calculating Lattice Energy Eel = к. Q 1 Q 2 d where, l К is a constant (8. 99 x 109 J-m/C 2) l Q 1 and Q 2 are the charges on the ions l d is the distance between their centers

l l l Therefore, the magnitude of the lattice energy is dependant upon: the charges on the particles (Q 1 and Q 2) the distance between the nuclei

Summary l For a given arrangement of ions, the lattice energy increases as the charges on the ions increase and as their radii decrease

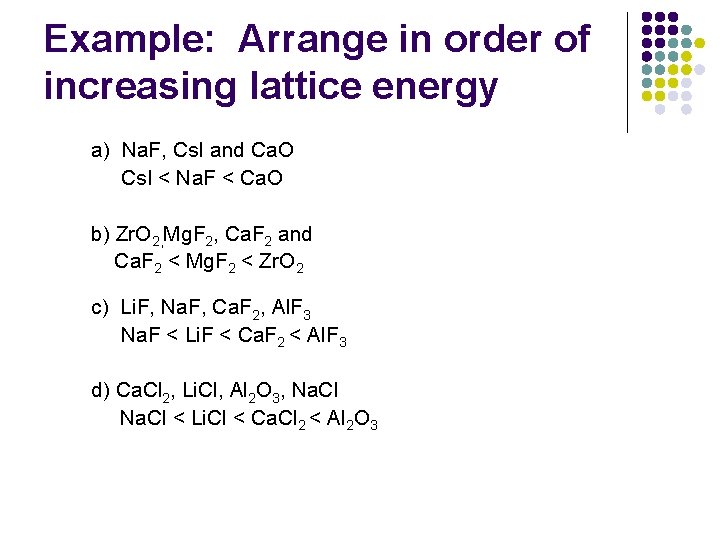
Example: Arrange in order of increasing lattice energy a) Na. F, Cs. I and Ca. O Cs. I < Na. F < Ca. O b) Zr. O 2, Mg. F 2, Ca. F 2 and Ca. F 2 < Mg. F 2 < Zr. O 2 c) Li. F, Na. F, Ca. F 2, Al. F 3 Na. F < Li. F < Ca. F 2 < Al. F 3 d) Ca. Cl 2, Li. Cl, Al 2 O 3, Na. Cl < Li. Cl < Ca. Cl 2 < Al 2 O 3

The Born-Haber Cycle l Lattice energy cannot be determined by experiment l It is calculated by envisioning the formation of an ionic compound occurring in steps (Hess’s Law) l Called the Born-Haber Cycle after Max Born and Fritz Haber (German scientists)


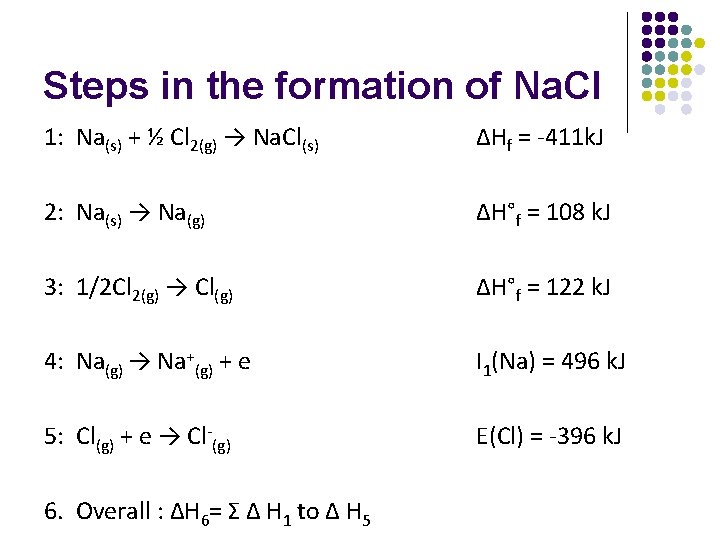
Steps in the formation of Na. Cl 1: Na(s) + ½ Cl 2(g) → Na. Cl(s) ∆Hf = -411 k. J 2: Na(s) → Na(g) ∆H°f = 108 k. J 3: 1/2 Cl 2(g) → Cl(g) ∆H°f = 122 k. J 4: Na(g) → Na+(g) + e I 1(Na) = 496 k. J 5: Cl(g) + e → Cl-(g) E(Cl) = -396 k. J 6. Overall : ΔH 6= Σ Δ H 1 to Δ H 5
 L a t t i c e
L a t t i c e What is energy physics
What is energy physics What is first ionization energy
What is first ionization energy Bond energies
Bond energies Law of specific nerve energies
Law of specific nerve energies Successive ionisation energies of calcium
Successive ionisation energies of calcium Bonding forces and energies
Bonding forces and energies European renewable energies federation
European renewable energies federation Reactants minus products bond energies
Reactants minus products bond energies We energies meter removal
We energies meter removal Four energies
Four energies Energies convencionals
Energies convencionals