2 Precipitation Gravimetry In precipitation gravimetry the analyte


![Example : in the determination of iron (III) [Fe 3+ ions] the precipitated form Example : in the determination of iron (III) [Fe 3+ ions] the precipitated form](https://slidetodoc.com/presentation_image_h2/b2cd72cba1bb94f60d3a8f9419dd286a/image-4.jpg)




- Slides: 16

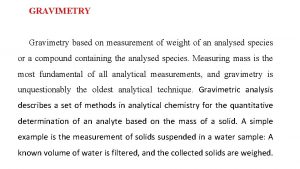
2 - Precipitation Gravimetry. In precipitation gravimetry, the analyte is converted to a sparingly soluble precipitate. This precipitate is then filtered, washed free of impurities, converted to a product of known composition by suitable heat treatment, and weighed.

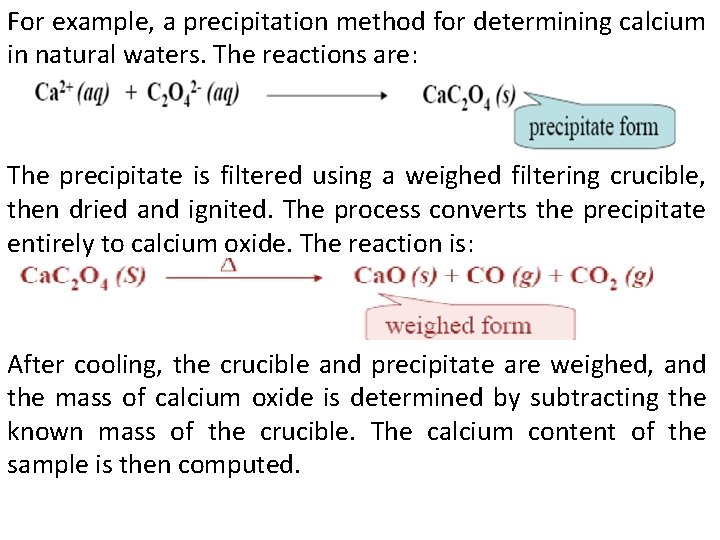
For example, a precipitation method for determining calcium in natural waters. The reactions are: The precipitate is filtered using a weighed filtering crucible, then dried and ignited. The process converts the precipitate entirely to calcium oxide. The reaction is: After cooling, the crucible and precipitate are weighed, and the mass of calcium oxide is determined by subtracting the known mass of the crucible. The calcium content of the sample is then computed.

Precipitated Form : is the name given to the compound precipitated from the solution by the action of suitable reagent. Weighed Form : is the name given to the compound which is weighed for determination of final results.
![Example in the determination of iron III Fe 3 ions the precipitated form Example : in the determination of iron (III) [Fe 3+ ions] the precipitated form](https://slidetodoc.com/presentation_image_h2/b2cd72cba1bb94f60d3a8f9419dd286a/image-4.jpg)
Example : in the determination of iron (III) [Fe 3+ ions] the precipitated form usually is Fe(OH)3 formed by the action of NH 4 OH solution while the weighed form is the anhydrous oxide (Fe 2 O 3) formed by ignition. Otherwise, in some cases the precipitated forms and the weighed forms are the same compound, for example : -

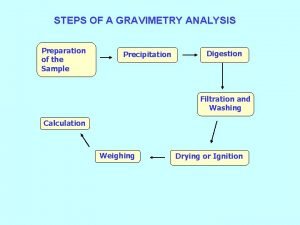
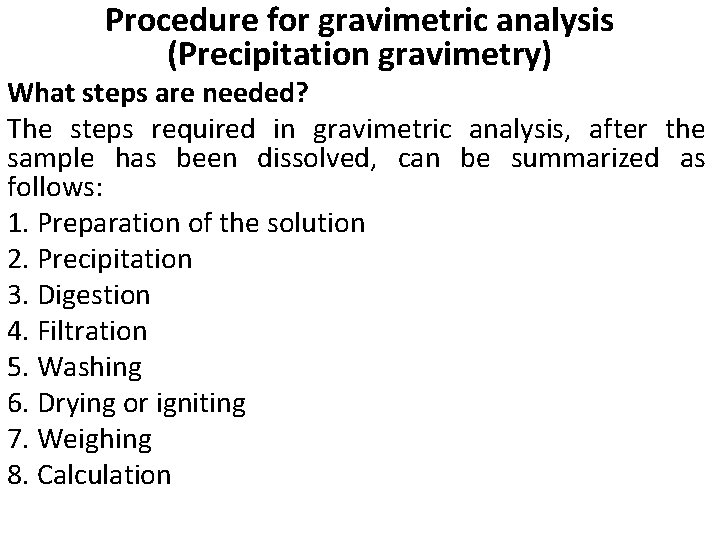
Procedure for gravimetric analysis (Precipitation gravimetry) What steps are needed? The steps required in gravimetric analysis, after the sample has been dissolved, can be summarized as follows: 1. Preparation of the solution 2. Precipitation 3. Digestion 4. Filtration 5. Washing 6. Drying or igniting 7. Weighing 8. Calculation

Properties of good precipitates 1. Easily filtered and washed free of contaminants. 2. Of sufficiently low solubility that no significant loss of the analyte occurs during filtration and washing. 3. Unreactive with constituents of the atmosphere 4. Of known chemical composition after it is dried or, if necessary, ignited.

Particle size and filterability of precipitates Why we prefer precipitates of large particles. Precipitates consisting of large particles are generally desirable for gravimetric work because large particles are usually: 1 - purer than fine particles, 2 - easy to filter, 3 - easy to wash and free of impurities.

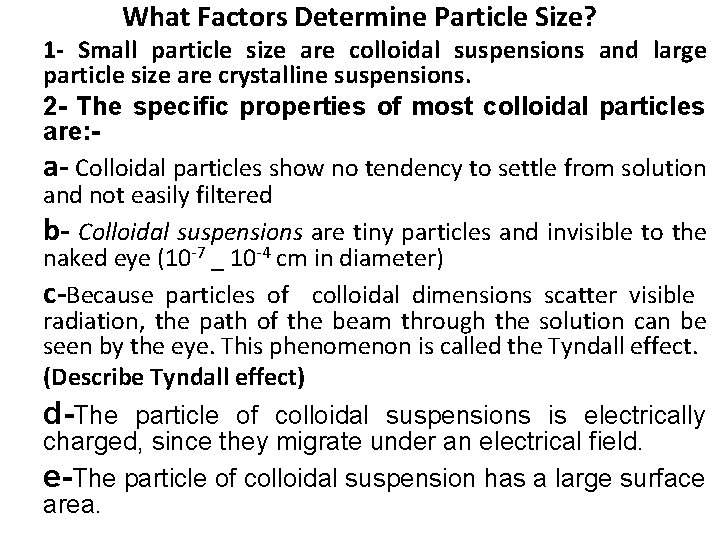
What Factors Determine Particle Size? 1 - Small particle size are colloidal suspensions and large particle size are crystalline suspensions. 2 - The specific properties of most colloidal particles are: a- Colloidal particles show no tendency to settle from solution and not easily filtered b- Colloidal suspensions are tiny particles and invisible to the naked eye (10 -7 _ 10 -4 cm in diameter) c-Because particles of colloidal dimensions scatter visible radiation, the path of the beam through the solution can be seen by the eye. This phenomenon is called the Tyndall effect. (Describe Tyndall effect) d-The particle of colloidal suspensions is electrically charged, since they migrate under an electrical field. e-The particle of colloidal suspension has a large surface area.

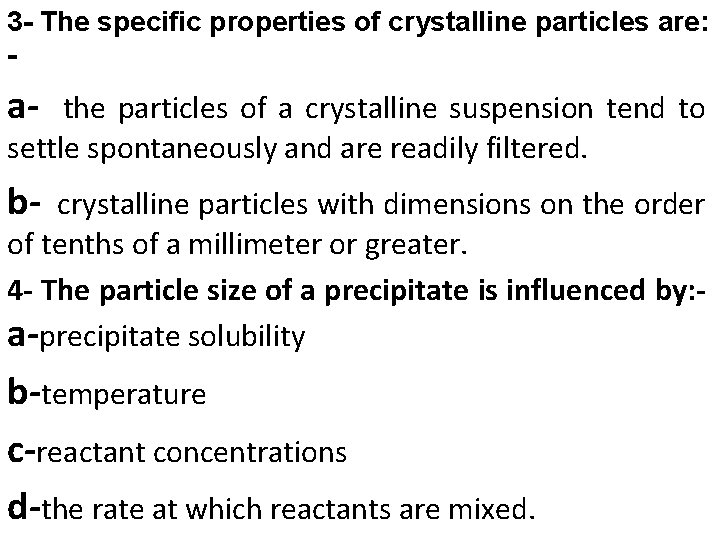
3 - The specific properties of crystalline particles are: - a- the particles of a crystalline suspension tend to settle spontaneously and are readily filtered. b- crystalline particles with dimensions on the order of tenths of a millimeter or greater. 4 - The particle size of a precipitate is influenced by: a-precipitate solubility b-temperature c-reactant concentrations d-the rate at which reactants are mixed.

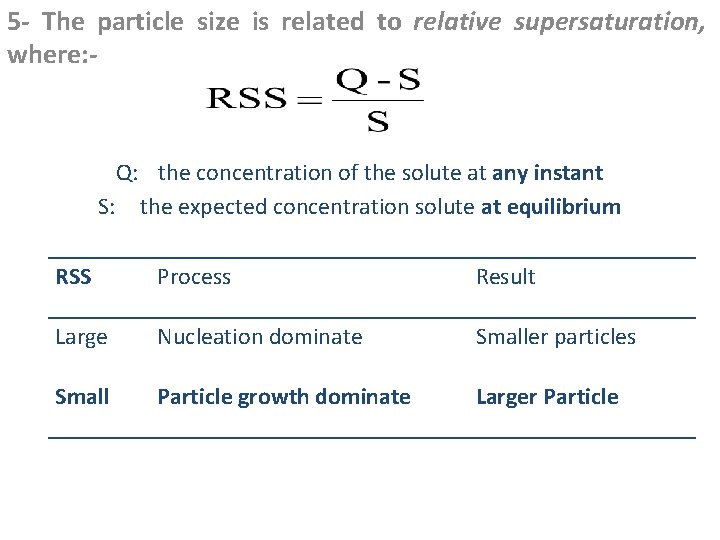
5 - The particle size is related to relative supersaturation, where: - Q: the concentration of the solute at any instant S: the expected concentration solute at equilibrium RSS Process Result Large Nucleation dominate Smaller particles Small Particle growth dominate Larger Particle

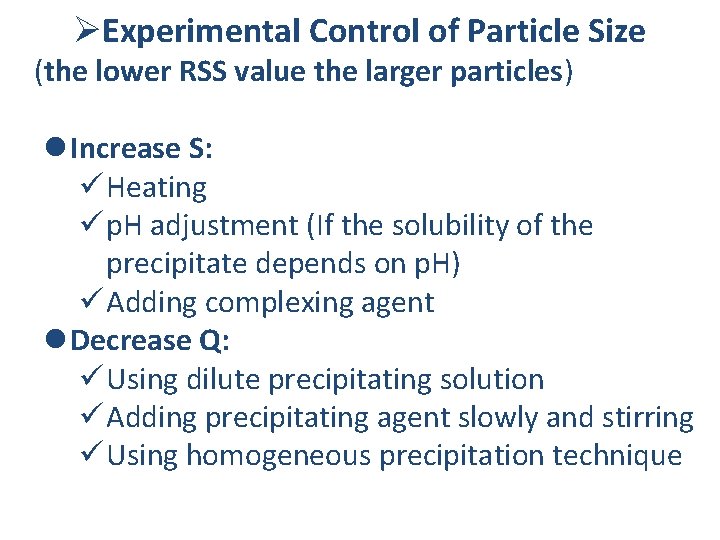
ØExperimental Control of Particle Size (the lower RSS value the larger particles) l Increase S: ü Heating ü p. H adjustment (If the solubility of the precipitate depends on p. H) ü Adding complexing agent l Decrease Q: ü Using dilute precipitating solution ü Adding precipitating agent slowly and stirring ü Using homogeneous precipitation technique

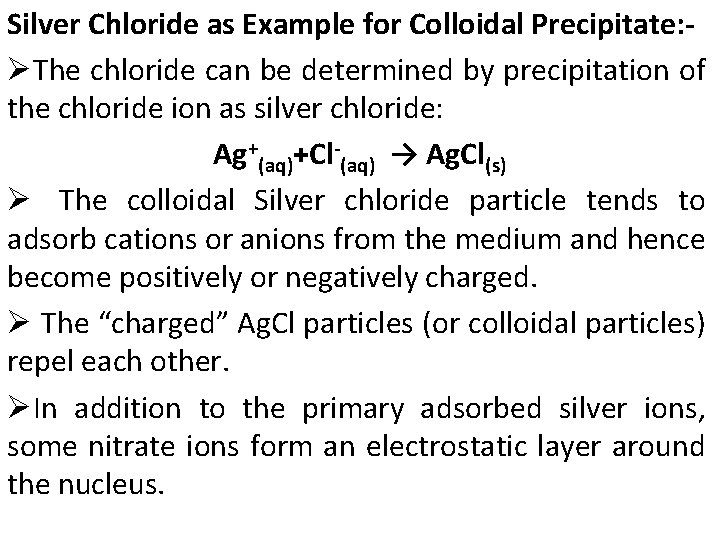
Colloidal Precipitate -- Colloidal suspensions are often stable for an indefinite period and are not desirable for gravimetric analysis because their particles are too small to be readily filtered. -- The stability of colloidal precipitates results from adsorption of ions to the colloidal particles.

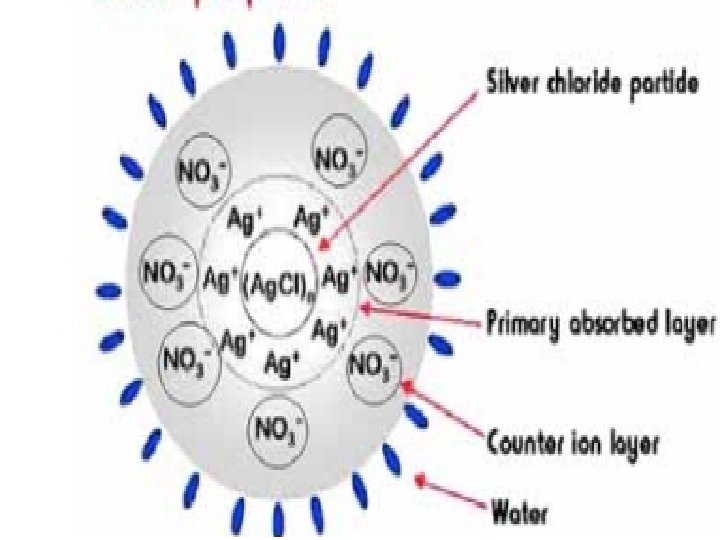
Silver Chloride as Example for Colloidal Precipitate: ØThe chloride can be determined by precipitation of the chloride ion as silver chloride: Ag+(aq)+Cl-(aq) → Ag. Cl(s) Ø The colloidal Silver chloride particle tends to adsorb cations or anions from the medium and hence become positively or negatively charged. Ø The “charged” Ag. Cl particles (or colloidal particles) repel each other. ØIn addition to the primary adsorbed silver ions, some nitrate ions form an electrostatic layer around the nucleus.


How do colloidal particle become charged or acquire charge? The charge on the colloidal particles may be due to (i) preferential adsorption of ions from the medium (ii)formation of electrical double layer • Adsorption is a process in which a substance (gas, liquid, or solid) condenses onto the surface of a solid • The electric double layer of a colloid consists of a layer of charge associated with the surface of the particles and a layer with a net opposite charge in the solution surrounding the particles

Describe how colloidal particles acquire charge by preferential adsorption of ions The colloidal particle tends to adsorb cations or anions from the medium and hence become positively or negatively charged.
 Carbohydrates
Carbohydrates Applications of uv visible spectroscopy
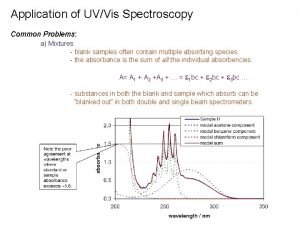
Applications of uv visible spectroscopy Gravimetric methods of analysis
Gravimetric methods of analysis Co precipitation and post precipitation
Co precipitation and post precipitation Co precipitation and post precipitation
Co precipitation and post precipitation Công của trọng lực
Công của trọng lực Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Gấu đi như thế nào
Gấu đi như thế nào Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Dot
Dot Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ độ dài liên kết
độ dài liên kết Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Lp html
Lp html Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống