CH 104 TITRATIONS WITH PERMANGANATE An analyte is






- Slides: 13

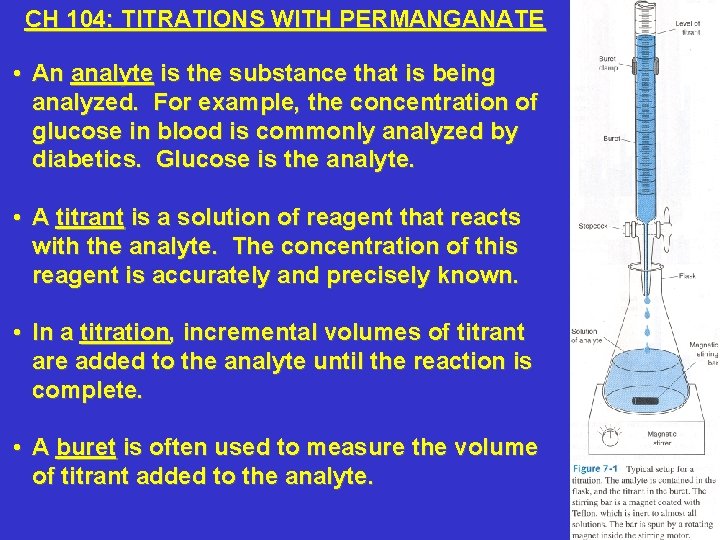
CH 104: TITRATIONS WITH PERMANGANATE • An analyte is the substance that is being analyzed. For example, the concentration of glucose in blood is commonly analyzed by diabetics. Glucose is the analyte. • A titrant is a solution of reagent that reacts with the analyte. The concentration of this reagent is accurately and precisely known. • In a titration, incremental volumes of titrant are added to the analyte until the reaction is complete. • A buret is often used to measure the volume of titrant added to the analyte.

REQUIREMENTS OF A TITRATION 1. The reaction must be stoichiometric. For example, the net ionic equation for the reaction of potassium permanganate (KMn. O 4) and sodium oxalate (Na 2 C 2 O 4) is quantitative. Exactly 2 moles of KMn. O 4 react with exactly 5 moles of Na 2 C 2 O 4. 1. 2 Mn. O 4–(aq)+ 16 H+(aq)+ 5 C 2 O 42–(aq) → 2 Mn 2+(aq)+ 8 H 2 O(l)+ 10 CO 2(g) 2. The reaction should be rapid. 3. The reaction should be specific; that is, there should be no competing reactions. Systematic error caused by interferences must be eliminated or reduced.

REQUIREMENTS OF A TITRATION 4. There should be a marked change when the reaction is complete. For example, this reaction is self-indicating. The titrant (KMn. O 4) is deep purple. The analyte (Na 2 C 2 O 4) and products (Mn 2+, H 2 O, and CO 2) are nearly colorless. The titration is done when the first fraction of a drop of excess Mn. O 4– changes the solution from nearly colorless to a faint and stable pink.

EQUIVALENCE POINT, END POINT, AND INDICATORS • The equivalence point occurs when the volume of titrant added to the analyte is the exact stoichiometric amount that is needed to bring the reaction to completion. • The end point occurs when the indicator changes color. • We want to measure the equivalence point. We actually measure the end point. • Obviously, the faint pink Mn. O 4– end point does not occur at the equivalence point. This end point occurs a fraction of a drop after the equivalence point. This error is small and can be corrected with a blank, or during standardization. • How would you use a blank to correct this error? • The volume of Mn. O 4– used to reach the end point during the titration of distilled water (a blank) is subtracted from all standards and all samples. • How would you standardize to correct this error? • All standards and all samples are titrated to the same end point. We will do this today.

EQUIVALENCE POINT, END POINT, AND INDICATORS Titration using Permanganate as a Self-Indicator • When do you stop adding titrant to the analyte? • At the end point.

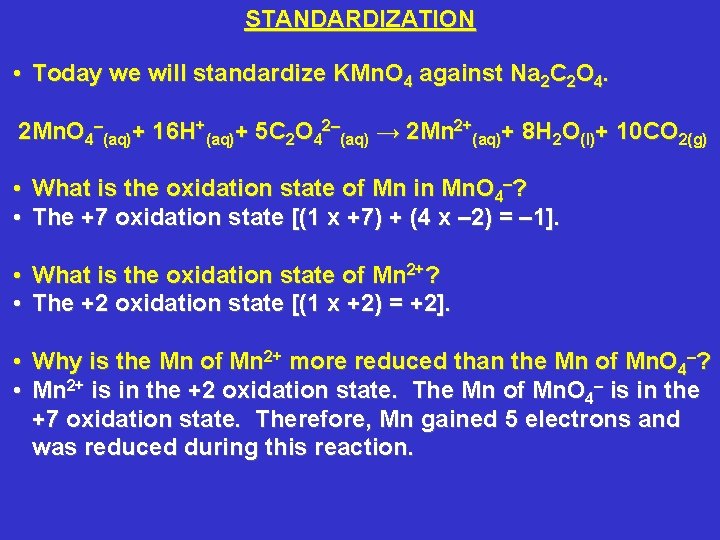
STANDARDIZATION • Today we will standardize KMn. O 4 against Na 2 C 2 O 4. 2 Mn. O 4–(aq)+ 16 H+(aq)+ 5 C 2 O 42–(aq) → 2 Mn 2+(aq)+ 8 H 2 O(l)+ 10 CO 2(g) • This is an oxidation-reduction reaction. That is, electrons are transferred from 1 reactant to another reactant. • Oxidation is a loss of an electron or electrons by an atom or group of atoms. • Reduction is a gain of an electron or electrons by an atom or group of atoms.

STANDARDIZATION • Today we will standardize KMn. O 4 against Na 2 C 2 O 4. 2 Mn. O 4–(aq)+ 16 H+(aq)+ 5 C 2 O 42–(aq) → 2 Mn 2+(aq)+ 8 H 2 O(l)+ 10 CO 2(g) • In this reaction, C 2 O 42– is oxidized to CO 2, and Mn. O 4– to reduced to Mn 2+. • What is the Lewis structure for C 2 O 42–? • What is the Lewis structure for CO 2? • Why is the C of CO 2 more oxidized than the C of C 2 O 42–? • The C in CO 2 is in the +4 oxidation state [(1 x +4) + (2 x – 2) = 0]. Each C in C 2 O 42– is in the +3 oxidation state [(2 x +3) + (4 x – 2) = – 2]. Therefore, each C lost 1 electron and was oxidized during this reaction.

STANDARDIZATION • Today we will standardize KMn. O 4 against Na 2 C 2 O 4. 2 Mn. O 4–(aq)+ 16 H+(aq)+ 5 C 2 O 42–(aq) → 2 Mn 2+(aq)+ 8 H 2 O(l)+ 10 CO 2(g) • What is the oxidation state of Mn in Mn. O 4–? • The +7 oxidation state [(1 x +7) + (4 x – 2) = – 1]. • What is the oxidation state of Mn 2+? • The +2 oxidation state [(1 x +2) = +2]. • Why is the Mn of Mn 2+ more reduced than the Mn of Mn. O 4–? • Mn 2+ is in the +2 oxidation state. The Mn of Mn. O 4– is in the +7 oxidation state. Therefore, Mn gained 5 electrons and was reduced during this reaction.

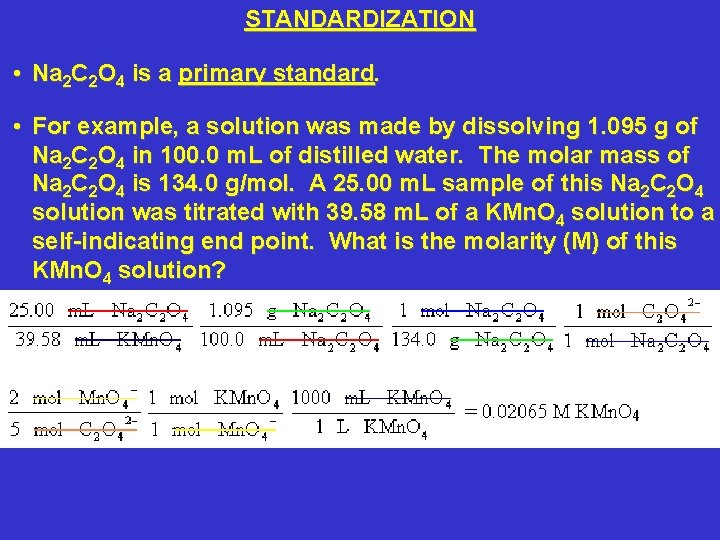
STANDARDIZATION • Na 2 C 2 O 4 is a primary standard. • For example, a solution was made by dissolving 1. 095 g of Na 2 C 2 O 4 in 100. 0 m. L of distilled water. The molar mass of Na 2 C 2 O 4 is 134. 0 g/mol. A 25. 00 m. L sample of this Na 2 C 2 O 4 solution was titrated with 39. 58 m. L of a KMn. O 4 solution to a self-indicating end point. What is the molarity (M) of this KMn. O 4 solution?

REQUIREMENTS OF A PRIMARY STANDARD 1. A primary standard should be 100. 00% pure; although a 0. 01% to 0. 02% impurity is tolerable if it is accurately known. 2. A primary standard should be stable at drying temperatures, and it should be stable indefinitely at room temperature. (A primary standard is always dried before weighing, unless it is a hydrate. ) 3. It should be readily available. 4. It should have a relatively large formula weight. Therefore, a relatively large mass of it will be weighed for titration. This will reduce error. • Explain this last point.

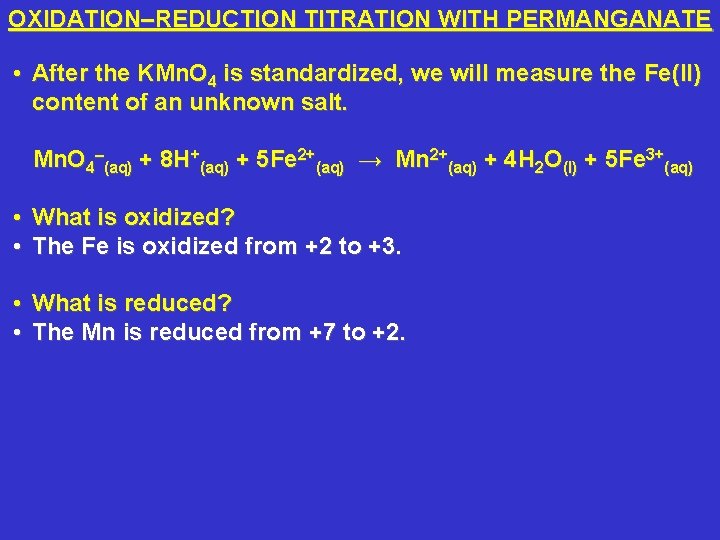
OXIDATION–REDUCTION TITRATION WITH PERMANGANATE • After the KMn. O 4 is standardized, we will measure the Fe(II) content of an unknown salt. Mn. O 4–(aq) + 8 H+(aq) + 5 Fe 2+(aq) → Mn 2+(aq) + 4 H 2 O(l) + 5 Fe 3+(aq) • What is oxidized? • The Fe is oxidized from +2 to +3. • What is reduced? • The Mn is reduced from +7 to +2.

SAFETY • Give at least 1 safety concern for the following procedure. • Using oxidizing agents (KMn. O 4), reducing agents (Na 2 C 2 O 4 and unknown Fe(II) salt), and acids (H 2 SO 4 and H 3 PO 4). • These are irritants. Wear your goggles at all times. Immediately clean all spills. If you do get either of these in your eye, immediately flush with water. • Your laboratory manual has an extensive list of safety procedures. Read and understand this section. • Ask your instructor if you ever have any questions about safety.

SOURCES • Christian, G. D. 1986. Analytical Chemistry, 3 rd ed. New York, NY: John Wiley & Sons, Inc. • Harris, D. C. 1999. Quantitative Chemical Analysis, 5 th ed. New York, NY: W. H. Freeman Company. • Mc. Murry, J. , R. C. Fay. 2004. Chemistry, 4 th ed. Upper Saddle River, NJ: Prentice Hall. • Petrucci, R. H. 1985. General Chemistry Principles and Modern Applications, 4 th ed. New York, NY: Macmillan Publishing Company.