2 Manipulation of the immune response Antigen specific














- Slides: 51

2. Manipulation of the immune response: Ø Antigen specific • Immunostimulation • Immunosuppression Ø Non-antigen specific -Immunostimulation -Immunosuppression Ø Immunomodulation

Targets of immunotherapies: elements of the immune response: cytokines, adhesion molecules, cell membrane molecules, receptors, antibodies Antibodies as drugs: specificity antigenicity humanized antibodies, „targeting”

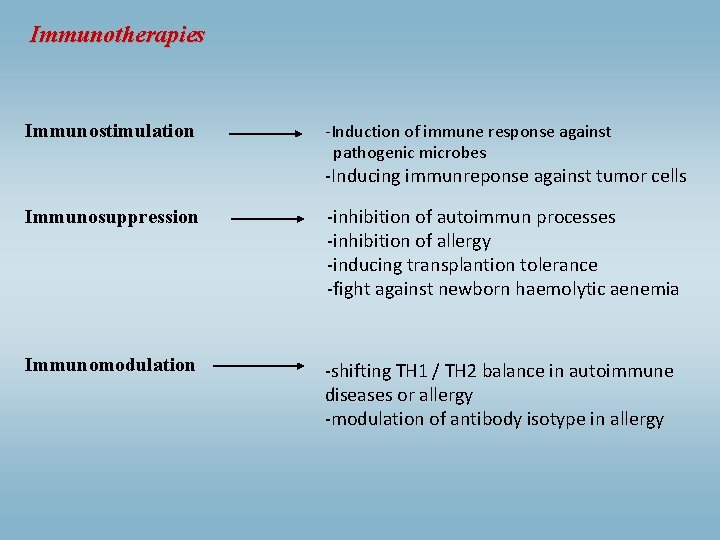
Immunotherapies Immunostimulation -Induction of immune response against pathogenic microbes -Inducing immunreponse against tumor cells Immunosuppression -inhibition of autoimmun processes -inhibition of allergy -inducing transplantion tolerance -fight against newborn haemolytic aenemia Immunomodulation -shifting TH 1 / TH 2 balance in autoimmune diseases or allergy -modulation of antibody isotype in allergy

Fighting against infections After infection ØTraditional drugs (antibiotics, anti-viral drugs) ØPassive immunization (antibodies, cells) ØAktive vaccination Before infection vaccination 1) increase the ratio of antigen specific cells 2) inducing specific immunological memory

IMMUNOSTIMULATION vaccination Infectious diseases, epidemics Edward Jenner, 1796, smallpox „A landmark was discovery of the germ theory, which included small parasites, bacteria and viruses. This theory was mainly based on the studies of Robert Koch (1843 -1910), Louis Pasteur (1822 -1895) and many others. ”

What will happen next. . . .


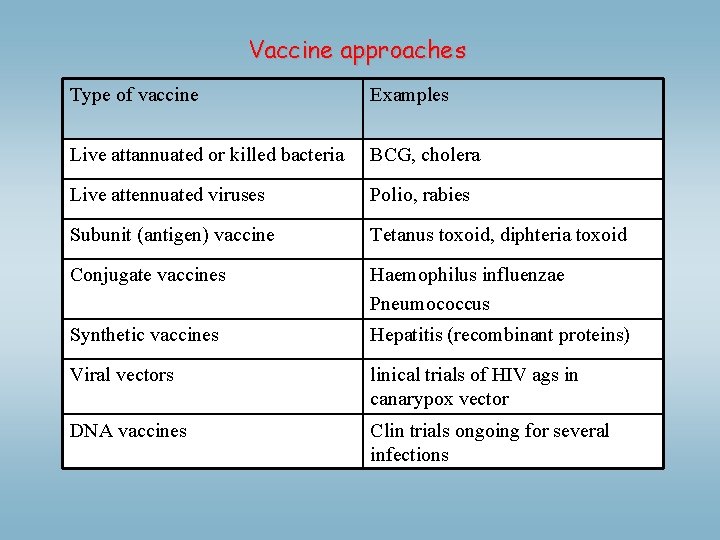
Vaccine approaches Type of vaccine Examples Live attannuated or killed bacteria BCG, cholera Live attennuated viruses Polio, rabies Subunit (antigen) vaccine Tetanus toxoid, diphteria toxoid Conjugate vaccines Haemophilus influenzae Pneumococcus Synthetic vaccines Hepatitis (recombinant proteins) Viral vectors linical trials of HIV ags in canarypox vector DNA vaccines Clin trials ongoing for several infections

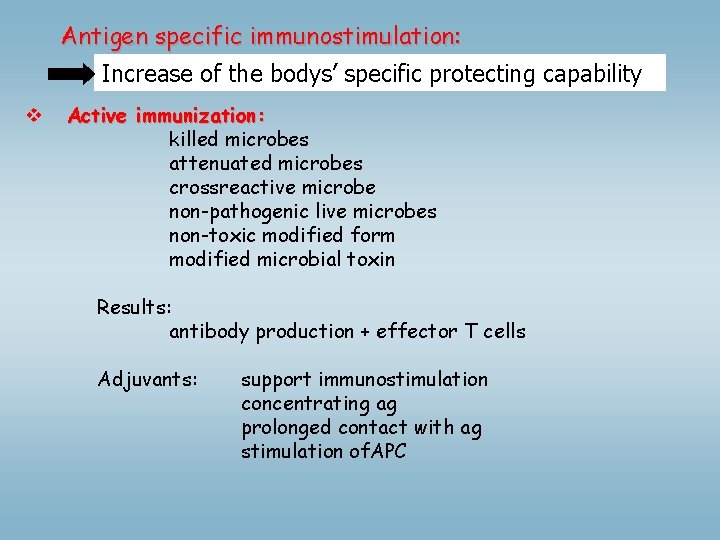
Antigen specific immunostimulation: Increase of the bodys’ specific protecting capability v Active immunization: killed microbes attenuated microbes crossreactive microbe non-pathogenic live microbes non-toxic modified form modified microbial toxin Results: antibody production + effector T cells Adjuvants: support immunostimulation concentrating ag prolonged contact with ag stimulation of. APC

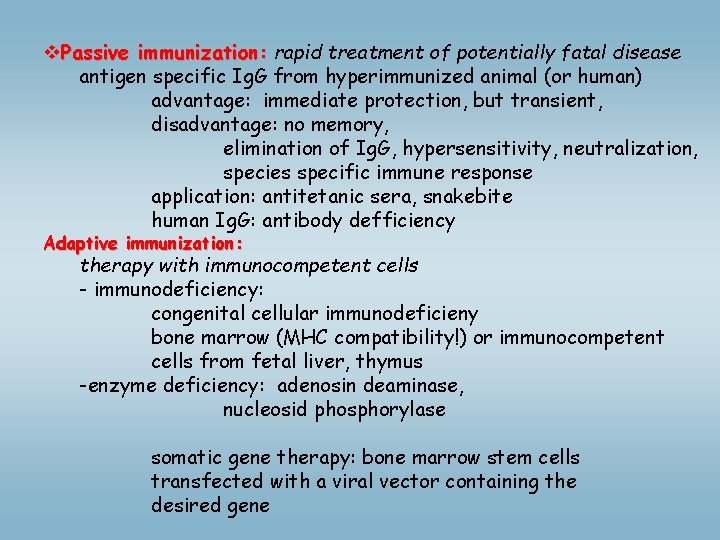
v. Passive immunization: rapid treatment of potentially fatal disease antigen specific Ig. G from hyperimmunized animal (or human) advantage: immediate protection, but transient, disadvantage: no memory, elimination of Ig. G, hypersensitivity, neutralization, species specific immune response application: antitetanic sera, snakebite human Ig. G: antibody defficiency Adaptive immunization: therapy with immunocompetent cells - immunodeficiency: congenital cellular immunodeficieny bone marrow (MHC compatibility!) or immunocompetent cells from fetal liver, thymus -enzyme deficiency: adenosin deaminase, nucleosid phosphorylase somatic gene therapy: bone marrow stem cells transfected with a viral vector containing the desired gene

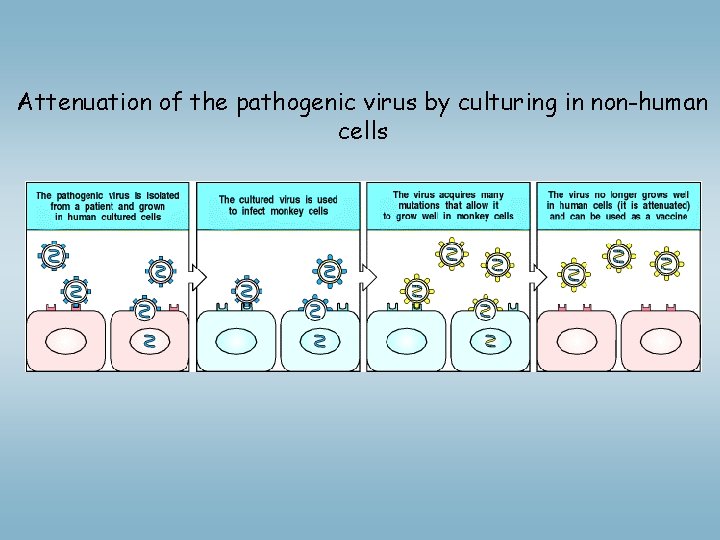
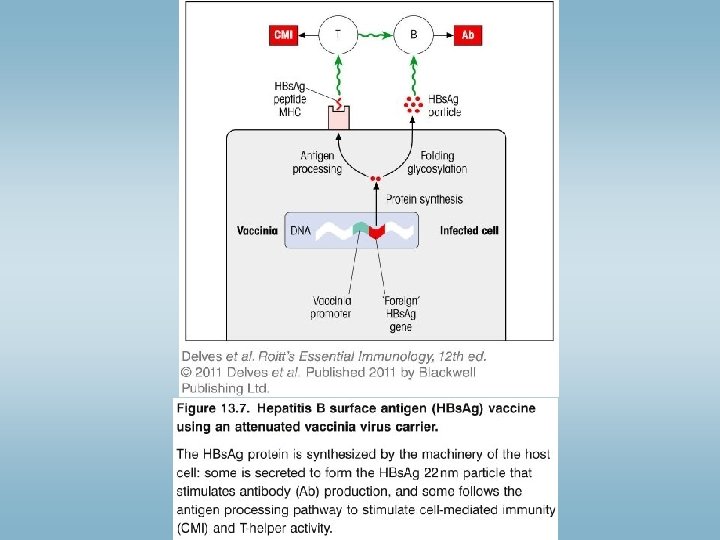
Vaccination – antigen specific immunostimulation Vaccine: against the microbe, or toxin produced by the microbe Live, attennuated virus are more efficient compared to killed virus (effector mechanisms, CD 8+T cells higher ) – but: risk! New techniques: Recombinant DNA technologies Immunization with dendritic cells – new type vaccines ØAttennuation of pathogenic microbes: Culturing virus in monkey cells mutations virus growth in monkey cells, but does not growth in human cells vaccine ØIn vitro mutagenesis: irreversible modification of virus gene influensa- changes every year - antigén shift directed mutagenezis

Attenuation of the pathogenic virus by culturing in non-human cells


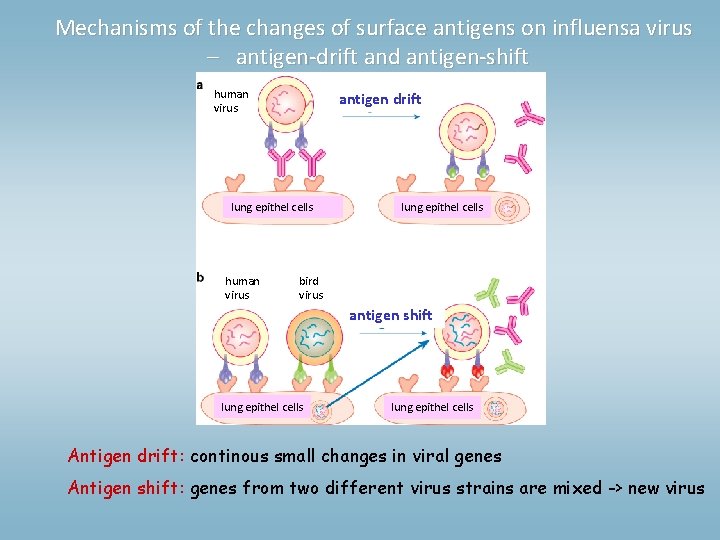
Mechanisms of the changes of surface antigens on influensa virus – antigen-drift and antigen-shift human virus antigen drift lung epithel cells human virus lung epithel cells bird virus antigen shift lung epithel cells Antigen drift: continous small changes in viral genes Antigen shift: genes from two different virus strains are mixed -> new virus

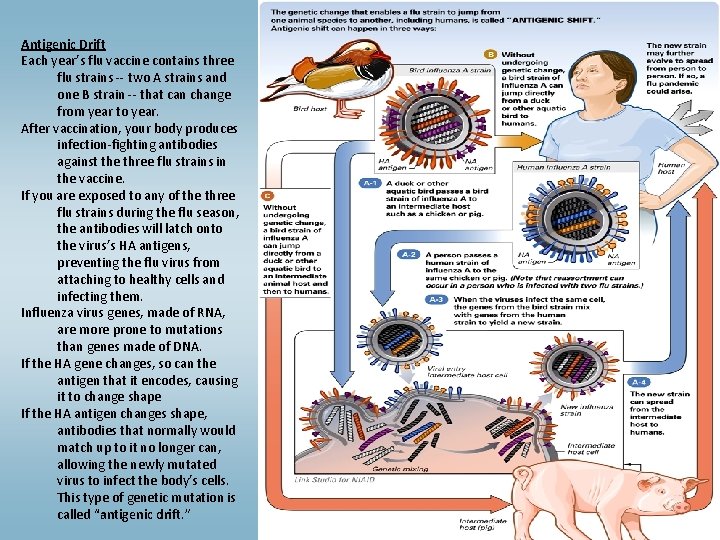
Antigenic Drift Each year’s flu vaccine contains three flu strains -- two A strains and one B strain -- that can change from year to year. After vaccination, your body produces infection-fighting antibodies against the three flu strains in the vaccine. If you are exposed to any of the three flu strains during the flu season, the antibodies will latch onto the virus’s HA antigens, preventing the flu virus from attaching to healthy cells and infecting them. Influenza virus genes, made of RNA, are more prone to mutations than genes made of DNA. If the HA gene changes, so can the antigen that it encodes, causing it to change shape If the HA antigen changes shape, antibodies that normally would match up to it no longer can, allowing the newly mutated virus to infect the body’s cells. This type of genetic mutation is called “antigenic drift. ”

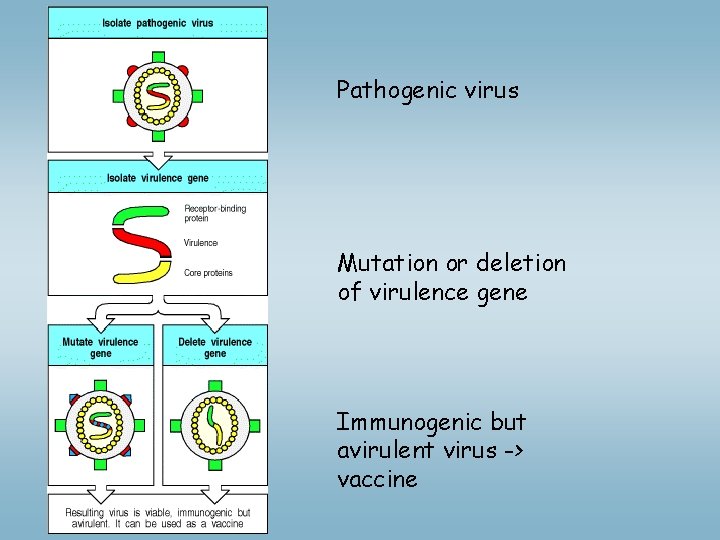
Pathogenic virus Mutation or deletion of virulence gene Immunogenic but avirulent virus -> vaccine

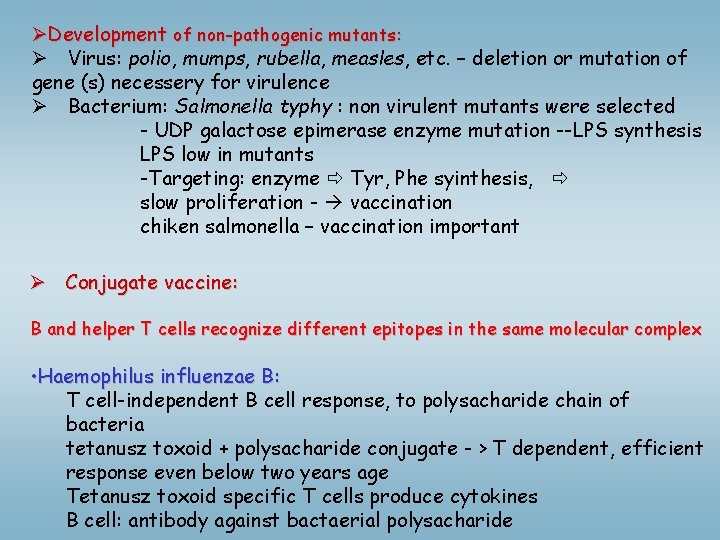
ØDevelopment of non-pathogenic mutants: Ø Virus: polio, mumps, rubella, measles, etc. – deletion or mutation of gene (s) necessery for virulence Ø Bacterium: Salmonella typhy : non virulent mutants were selected - UDP galactose epimerase enzyme mutation --LPS synthesis LPS low in mutants -Targeting: enzyme Tyr, Phe syinthesis, slow proliferation - vaccination chiken salmonella – vaccination important Ø Conjugate vaccine: B and helper T cells recognize different epitopes in the same molecular complex • Haemophilus influenzae B: T cell-independent B cell response, to polysacharide chain of bacteria tetanusz toxoid + polysacharide conjugate - > T dependent, efficient response even below two years age Tetanusz toxoid specific T cells produce cytokines B cell: antibody against bactaerial polysacharide

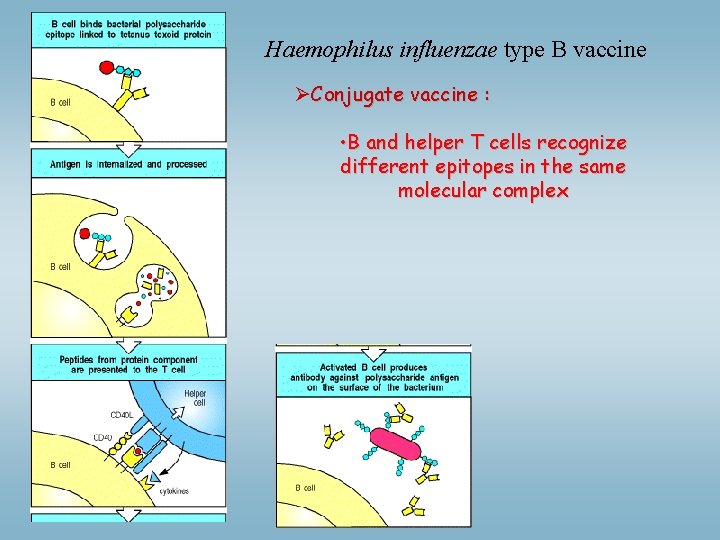
Haemophilus influenzae type B vaccine ØConjugate vaccine : • B and helper T cells recognize different epitopes in the same molecular complex



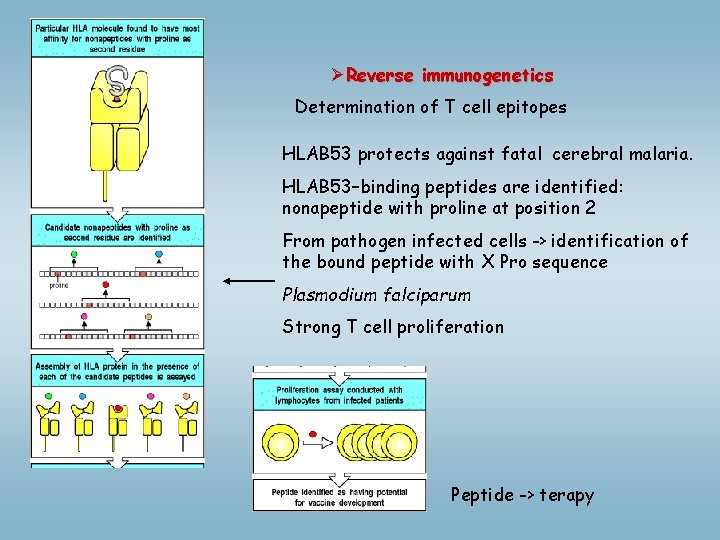
ØReverse immunogenetics Determination of T cell epitopes HLAB 53 protects against fatal cerebral malaria. HLAB 53–binding peptides are identified: nonapeptide with proline at position 2 From pathogen infected cells -> identification of the bound peptide with X Pro sequence Plasmodium falciparum Strong T cell proliferation Peptide -> terapy

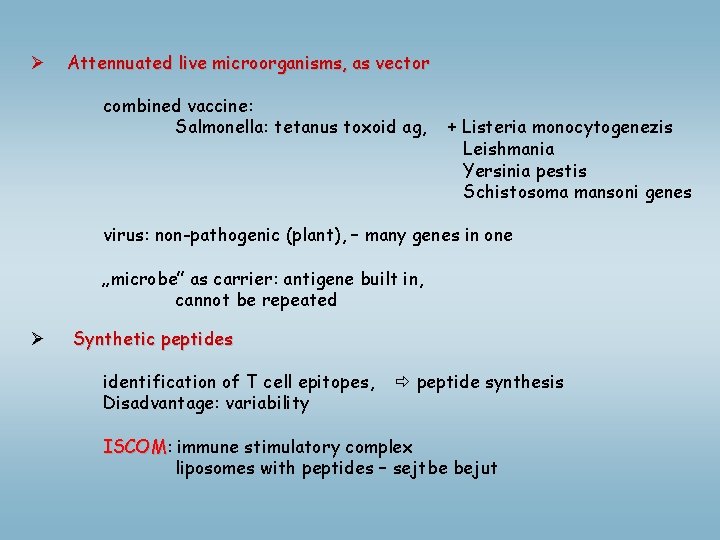
Ø Attennuated live microorganisms, as vector combined vaccine: Salmonella: tetanus toxoid ag, + Listeria monocytogenezis Leishmania Yersinia pestis Schistosoma mansoni genes Ø virus: non-pathogenic (plant), – many genes in one „microbe” as carrier: antigene built in, cannot be repeated Synthetic peptides identification of T cell epitopes, Disadvantage: variability peptide synthesis ISCOM: ISCOM immune stimulatory complex liposomes with peptides – sejtbe bejut

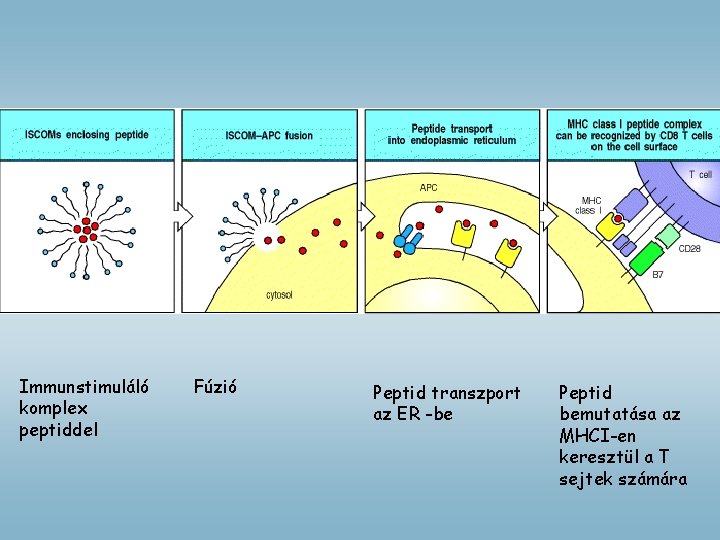
Immunstimuláló komplex peptiddel Fúzió Peptid transzport az ER -be Peptid bemutatása az MHCI-en keresztül a T sejtek számára




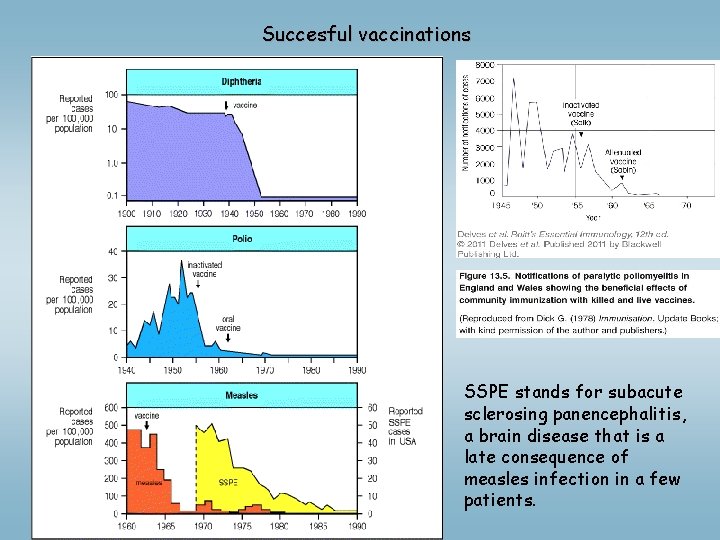
Succesful vaccinations SSPE stands for subacute sclerosing panencephalitis, a brain disease that is a late consequence of measles infection in a few patients.






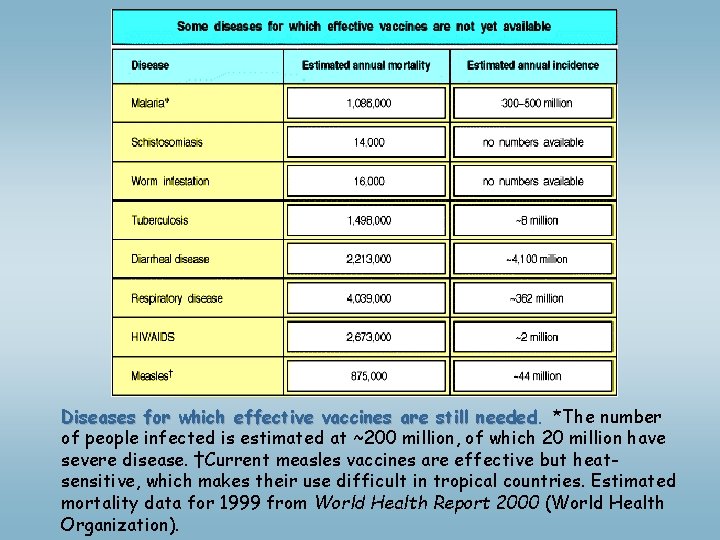
Diseases for which effective vaccines are still needed *The number of people infected is estimated at ~200 million, of which 20 million have severe disease. †Current measles vaccines are effective but heatsensitive, which makes their use difficult in tropical countries. Estimated mortality data for 1999 from World Health Report 2000 (World Health Organization).

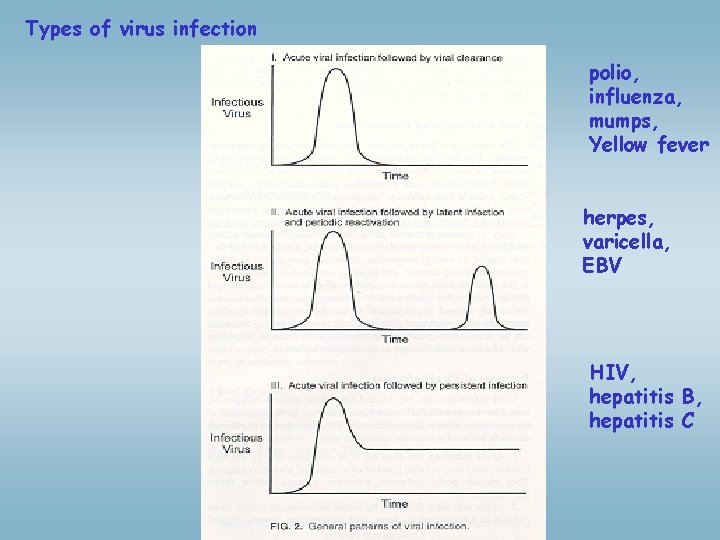
Types of virus infection polio, influenza, mumps, Yellow fever herpes, varicella, EBV HIV, hepatitis B, hepatitis C

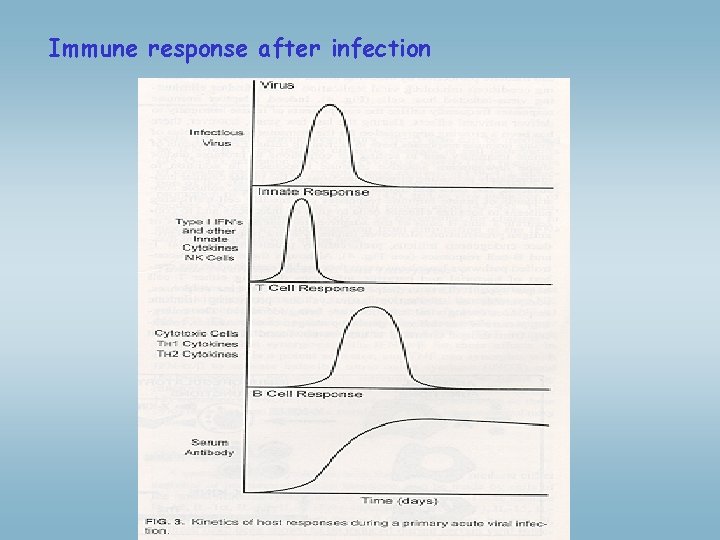
Immune response after infection

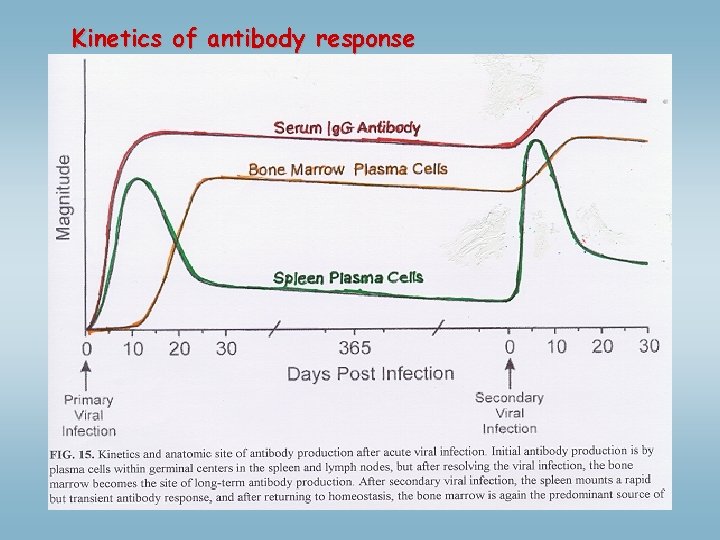
Kinetics of antibody response

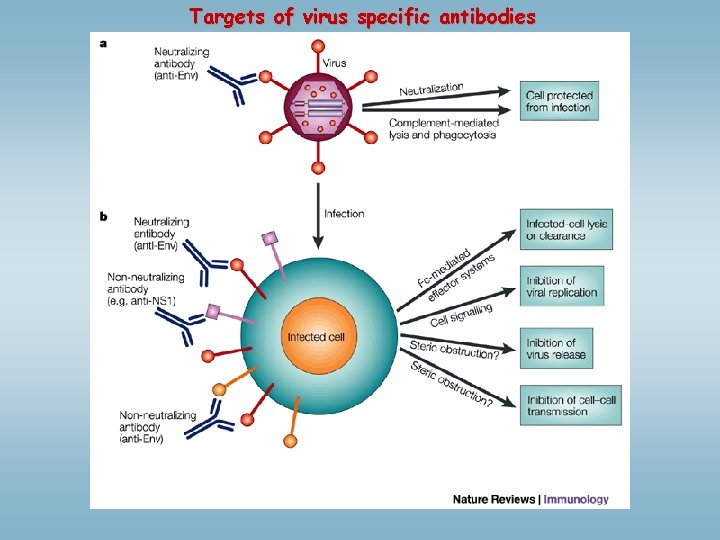
Targets of virus specific antibodies

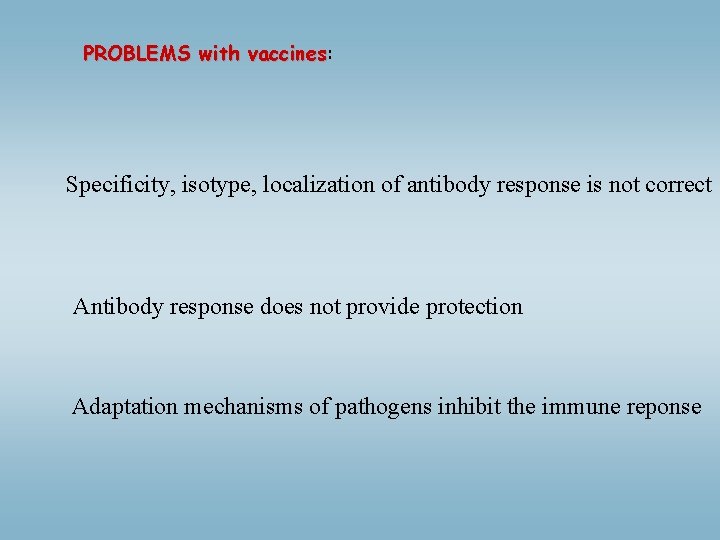
PROBLEMS with vaccines: vaccines Specificity, isotype, localization of antibody response is not correct Antibody response does not provide protection Adaptation mechanisms of pathogens inhibit the immune reponse

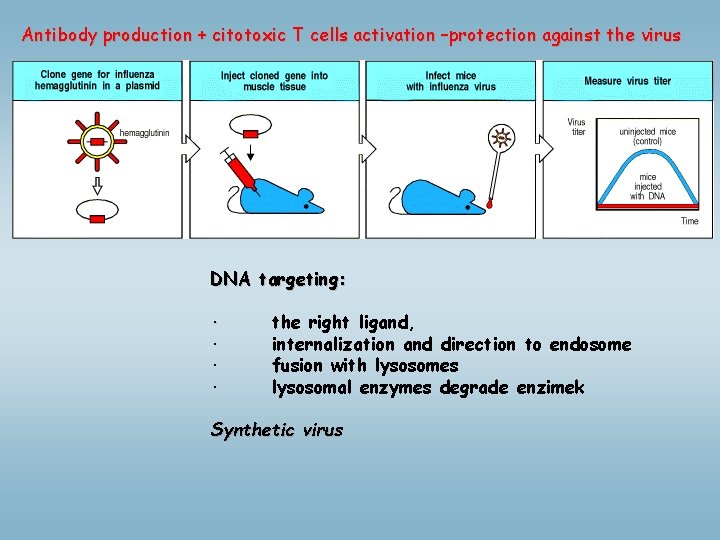
Antibody production + citotoxic T cells activation –protection against the virus DNA targeting: · the right ligand, · internalization and direction to endosome · fusion with lysosomes · lysosomal enzymes degrade enzimek Synthetic virus

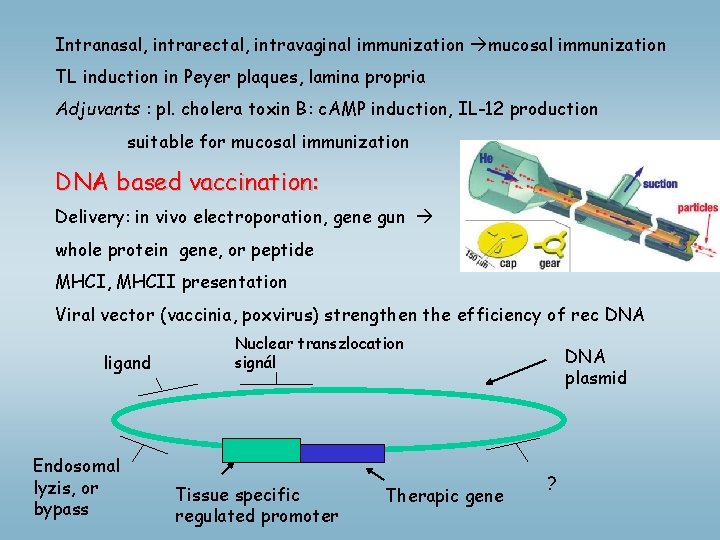
Intranasal, intrarectal, intravaginal immunization mucosal immunization TL induction in Peyer plaques, lamina propria Adjuvants : pl. cholera toxin B: c. AMP induction, IL-12 production suitable for mucosal immunization DNA based vaccination: Delivery: in vivo electroporation, gene gun whole protein gene, or peptide MHCI, MHCII presentation Viral vector (vaccinia, poxvirus) strengthen the efficiency of rec DNA ligand Endosomal lyzis, or bypass Nuclear transzlocation signál Tissue specific regulated promoter Therapic gene DNA plasmid ?


Vaccine design epitope adjuvant delivery, site of delivery

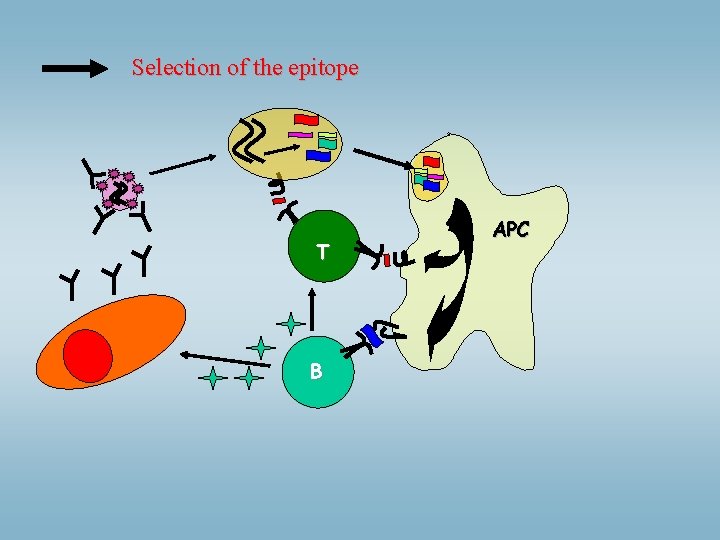
Selection of the epitope T B APC

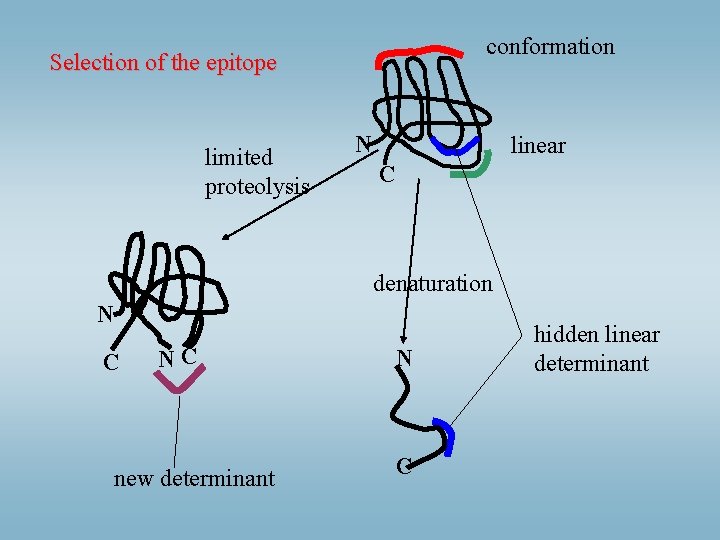
conformation Selection of the epitope limited proteolysis N linear C denaturation N C NC new determinant N C hidden linear determinant

Strategies to develop new generation of vaccines Virus – do not express suitable T cell epitopes evolution) (selection during Tumor – suitable T cells are deleted „Epitope enhancement” Ø increase peptide- MHC binding affinity - MHCII –TH cells repair of anchoring aa. Ø combinatorial peptide libraries Ø peptide-MHC complex – increase TCR- binding affinity : activation of both small and large affinity T cells epitope increase the number of T cells that recognize tumor Ø increase TCR crossreactivity: peptid chimera – recognition of different virus strains

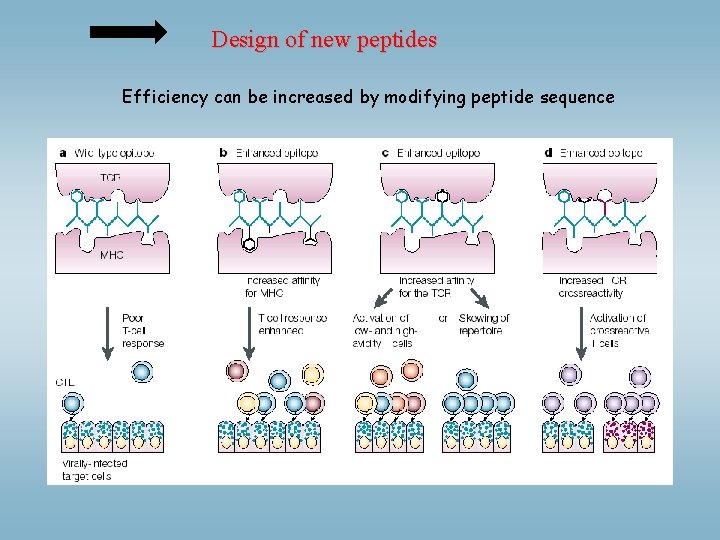
Design of new peptides Efficiency can be increased by modifying peptide sequence

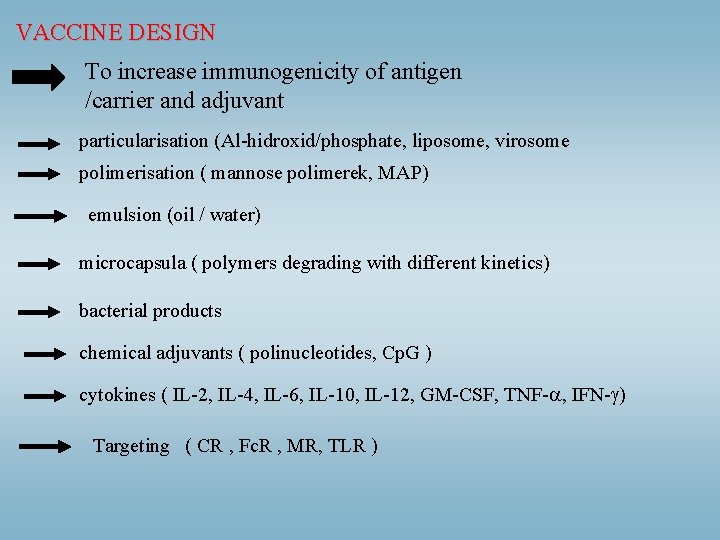
VACCINE DESIGN To increase immunogenicity of antigen /carrier and adjuvant particularisation (Al-hidroxid/phosphate, liposome, virosome polimerisation ( mannose polimerek, MAP) emulsion (oil / water) microcapsula ( polymers degrading with different kinetics) bacterial products chemical adjuvants ( polinucleotides, Cp. G ) cytokines ( IL-2, IL-4, IL-6, IL-10, IL-12, GM-CSF, TNF- , IFN- ) Targeting ( CR , Fc. R , MR, TLR )

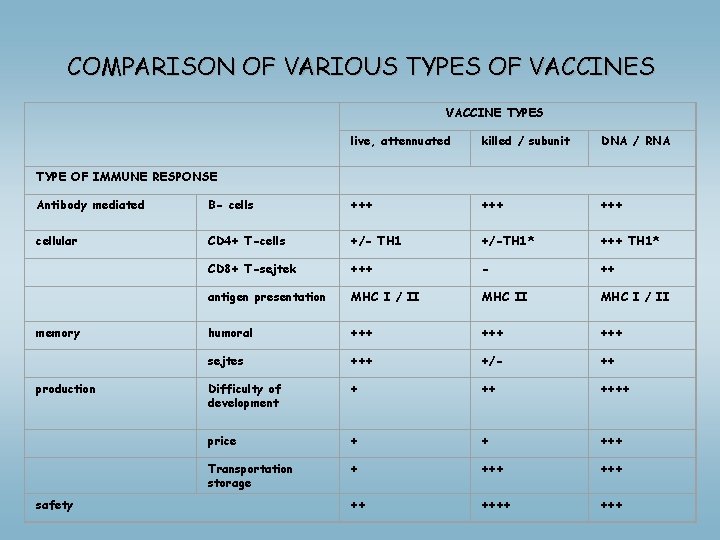
COMPARISON OF VARIOUS TYPES OF VACCINES VACCINE TYPES live, attennuated killed / subunit DNA / RNA TYPE OF IMMUNE RESPONSE Antibody mediated B- cells +++ +++ cellular CD 4+ T-cells +/- TH 1 +/-TH 1* +++ TH 1* CD 8+ T-sejtek +++ - ++ antigen presentation MHC I / II memory humoral +++ +++ sejtes +++ +/- ++ production Difficulty of development + ++ ++++ price + + +++ Transportation storage + +++ ++ +++ safety

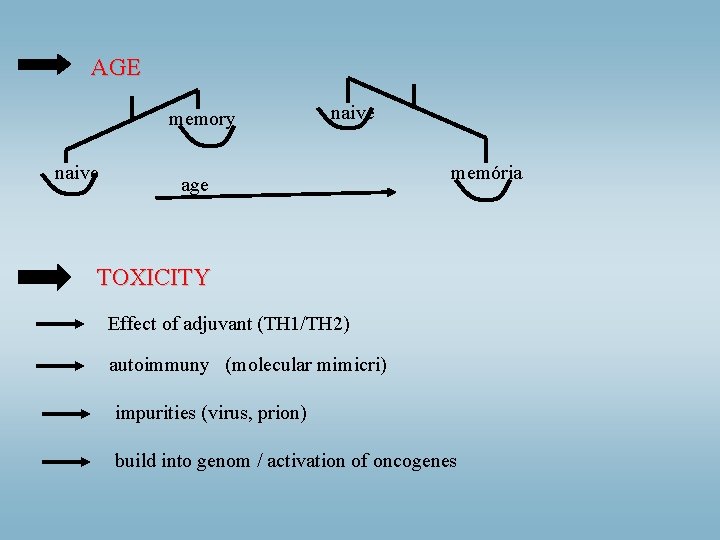
AGE memory naive age memória TOXICITY Effect of adjuvant (TH 1/TH 2) autoimmuny (molecular mimicri) impurities (virus, prion) build into genom / activation of oncogenes

The efficient vaccine: - safe –can be applied to everyone including children - efficient to protect against infection or disease (less efficient) - protection for life long – memory - induce neutralizing antibodies - induce T cell response - stabile, cheap, no/few side effect - easy applicable (oralis vaccine, e. g. Sabin dropp) - acceptable and applicable everywhere (developing world) Adjuvants: non-specific signal, stimulation of APC cytokine induction, antigén-depo: slow felszívódás aluminium hidroxid, or oil emulsion. mixed vaccines - one can activate the other ( e. g. Bordatella Pertussis+ tetanus+difteria) cytokines : IL-12 - TH 1 response The way of immunization Important: the site of infection/ immunizations Oral vaccines – the role of mucosa (MALT)

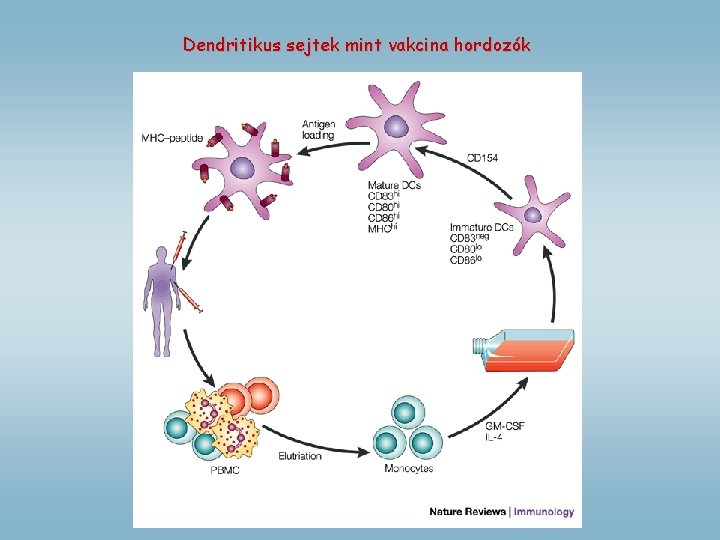
Dendritikus sejtek mint vakcina hordozók
 Primary immune response and secondary immune response
Primary immune response and secondary immune response E coli treatment
E coli treatment Primary immune response
Primary immune response Cellular immune response
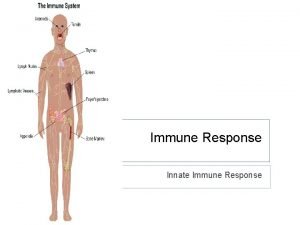
Cellular immune response Difference between innate and learned behavior
Difference between innate and learned behavior Unsaturated alcohol crossword clue
Unsaturated alcohol crossword clue Vaccinations help prepare the body to fight invasions of
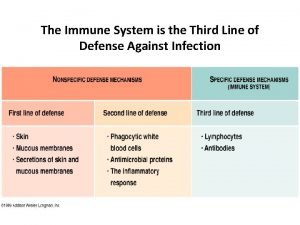
Vaccinations help prepare the body to fight invasions of 1st 2nd and 3rd line of defense immune system
1st 2nd and 3rd line of defense immune system Primary and secondary immune response
Primary and secondary immune response Third line of defense immune system
Third line of defense immune system Biosimilar study
Biosimilar study Autospecificity
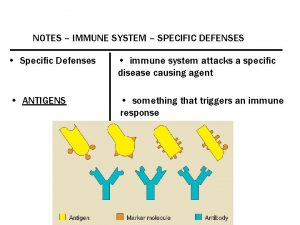
Autospecificity What is antigen in blood
What is antigen in blood Antigen-antikroppsreaktion
Antigen-antikroppsreaktion Mendelian
Mendelian Precipitation test
Precipitation test Characteristics of antigens
Characteristics of antigens Reza curtmola
Reza curtmola Cell response
Cell response Parham
Parham Flow cytometry voltration
Flow cytometry voltration Non-self antigen
Non-self antigen Blood type antigen antibody chart
Blood type antigen antibody chart Immune system definition
Immune system definition Toxin neutralization test
Toxin neutralization test Antibody
Antibody Interaksi antigen antibodi
Interaksi antigen antibodi Immune defintion
Immune defintion Antigen binding site
Antigen binding site Precipitation curve immunology
Precipitation curve immunology Specific volume is an abstract number
Specific volume is an abstract number Specific gravity formula
Specific gravity formula Natural and forced response
Natural and forced response Natural and forced response
Natural and forced response Immune effector cells
Immune effector cells Chapter 24 the immune and lymphatic systems and cancer
Chapter 24 the immune and lymphatic systems and cancer What is the third line of defense in the immune system
What is the third line of defense in the immune system 1what's the purpose of the body's immune system?
1what's the purpose of the body's immune system? Immune complex
Immune complex Dr patrick kormann
Dr patrick kormann Immune complex glomerulonephritis
Immune complex glomerulonephritis Chapter 35 immune system and disease
Chapter 35 immune system and disease Immune complex
Immune complex Thymus
Thymus Ctl
Ctl Phagocitize
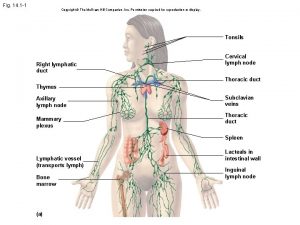
Phagocitize Lesson 12.1 lymphatic ducts and vessels
Lesson 12.1 lymphatic ducts and vessels Immune reconstitution inflammatory syndrome
Immune reconstitution inflammatory syndrome Flow chart
Flow chart Immune system structure
Immune system structure What is the first line of defense
What is the first line of defense Overreactions of the immune system
Overreactions of the immune system