1 The Atmosphere and Atmospheric Ozone Dr Paul
































- Slides: 62

1 The Atmosphere and Atmospheric Ozone Dr. Paul A. Newman http: //code 916. gsfc. nasa. gov/People/Newman/ NASA’s Goddard Space Flight Center 2005 NASA Earth System Science Teacher Workshop NASA GSFC April 22, 2005 Feb. 2, 2005

2 What are the main issues in atmospheric physics? • Ozone depletion • Atmospheric pollution • Climate change All 3 issues are related to changing atmospheric composition Feb. 2, 2005

3 Outline • Atmospheric Basics & Solar Radiation • Ozone: basics and photochemistry • Ozone loss in the atmosphere • Summary • Educational activities Feb. 2, 2005

4 Atmospheric Basics Feb. 2, 2005

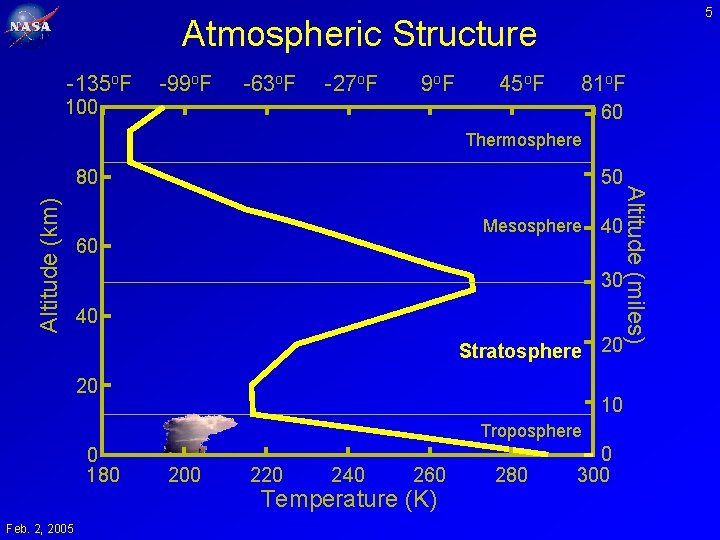
5 Atmospheric Structure -135 o. F -99 o. F -63 o. F -27 o. F 9 o. F 45 o. F 81 o. F 100 60 Thermosphere Altitude (km) 50 Mesosphere 60 40 30 40 Stratosphere 20 20 10 Troposphere 0 180 Feb. 2, 2005 200 220 240 260 Temperature (K) 280 0 300 Altitude (miles) 80

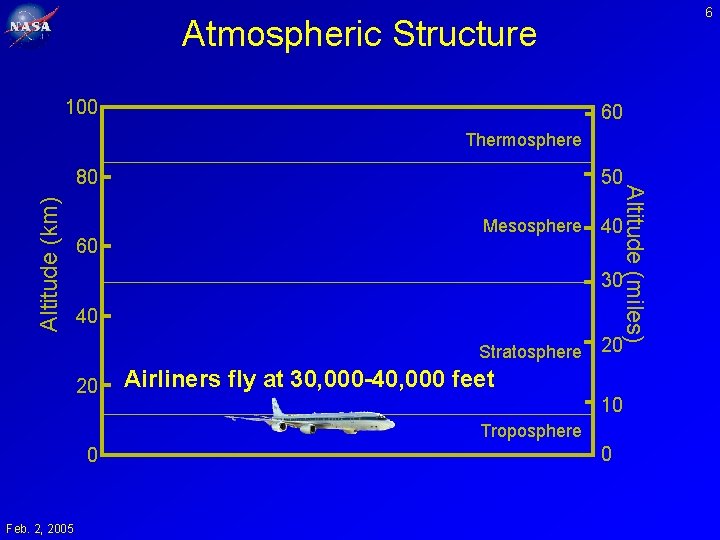
6 Atmospheric Structure 100 60 Thermosphere Altitude (km) 60 50 Mesosphere Altitude (miles) 80 40 30 40 Stratosphere 20 20 Airliners fly at 30, 000 -40, 000 feet 10 Troposphere 0 Feb. 2, 2005 0

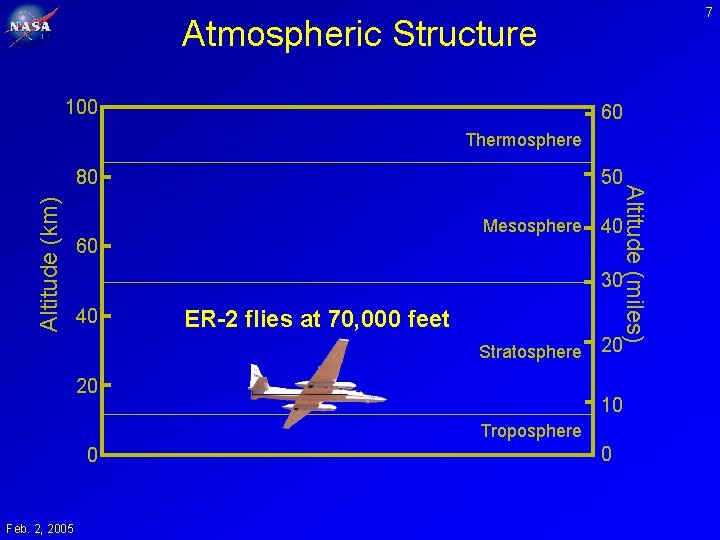
7 Atmospheric Structure 100 60 Thermosphere Altitude (km) 50 Mesosphere 60 Altitude (miles) 80 40 30 40 ER-2 flies at 70, 000 feet Stratosphere 20 20 10 Troposphere 0 Feb. 2, 2005 0

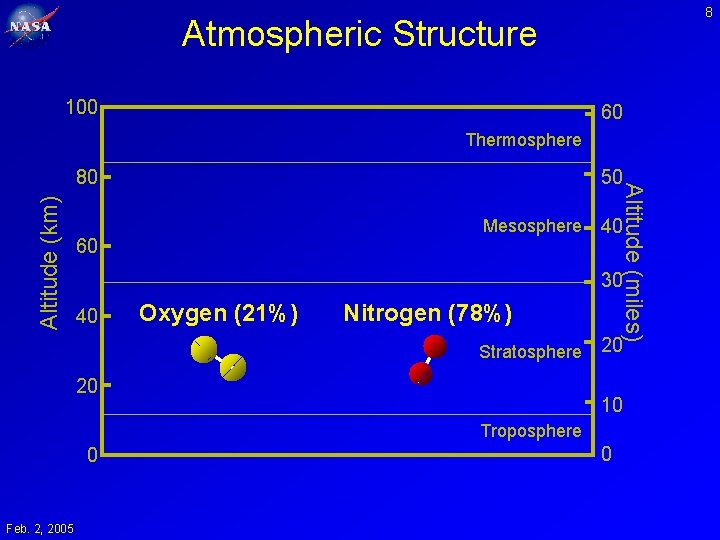
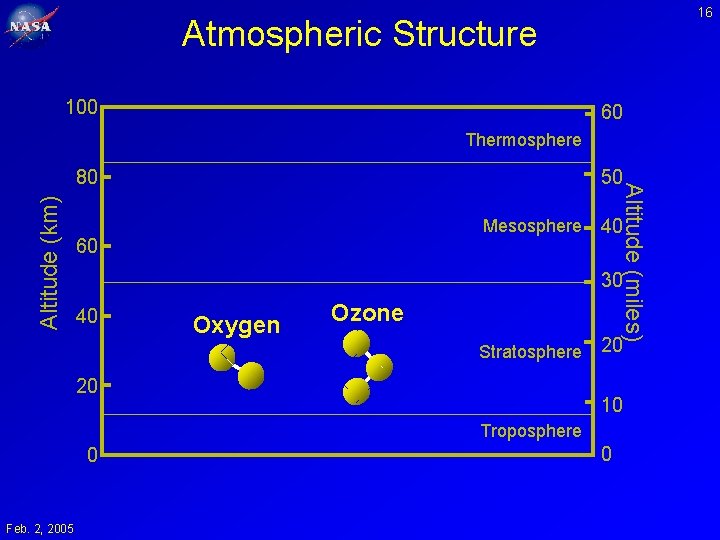
8 Atmospheric Structure 100 60 Thermosphere Altitude (km) 50 Mesosphere 60 Altitude (miles) 80 40 30 40 Oxygen (21%) Nitrogen (78%) Stratosphere 20 20 10 Troposphere 0 Feb. 2, 2005 0

9 Atmospheric Composition • • Nitrogen Oxygen Argon Water • CO 2 • O 3 Feb. 2, 2005 0. 781 0. 209 0. 014 (tropics) 0. 002 (poles) 0. 000004 (stratosphere) 0. 000360 0. 0000100 (stratosphere) 0. 0000001 (troposphere)

10 Solar Radiation: The photochemistry driver Feb. 2, 2005

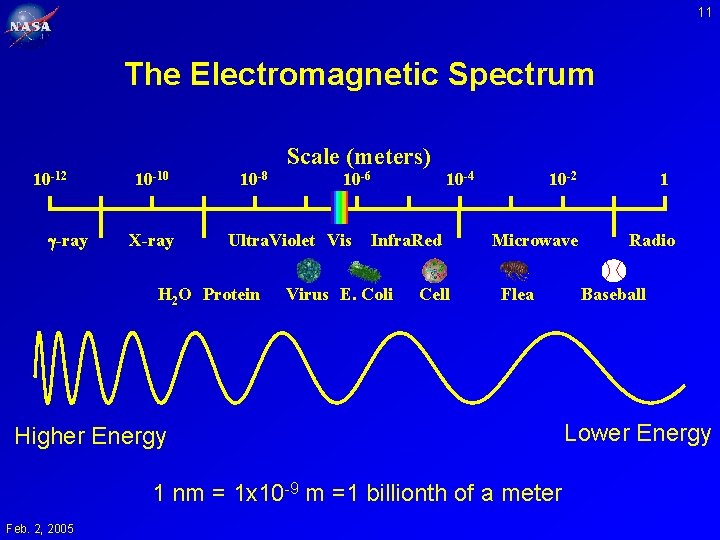
11 The Electromagnetic Spectrum 10 -12 -ray 10 -10 X-ray 10 -8 Scale (meters) 10 -6 Ultra. Violet Vis H 2 O Protein 10 -4 Infra. Red Virus E. Coli Cell 10 -2 Microwave Flea Higher Energy 1 nm = 1 x 10 -9 m =1 billionth of a meter Feb. 2, 2005 1 Radio Baseball Lower Energy

12 UV IR Solar Energy Outside Earth’s Atmosphere (from space) Solar Energy at Earth’s Surface Feb. 2, 2005 1 m = 1 x 10 -6 m =1 millionth of a meter

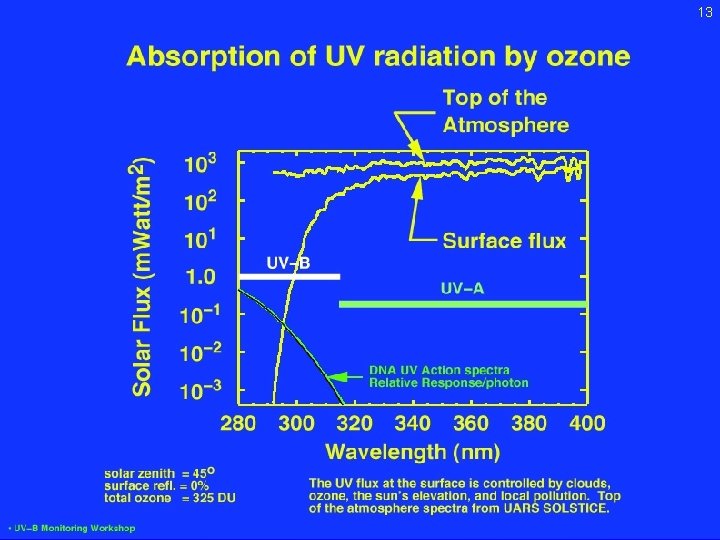
13 Absorption of UV by ozone Feb. 2, 2005

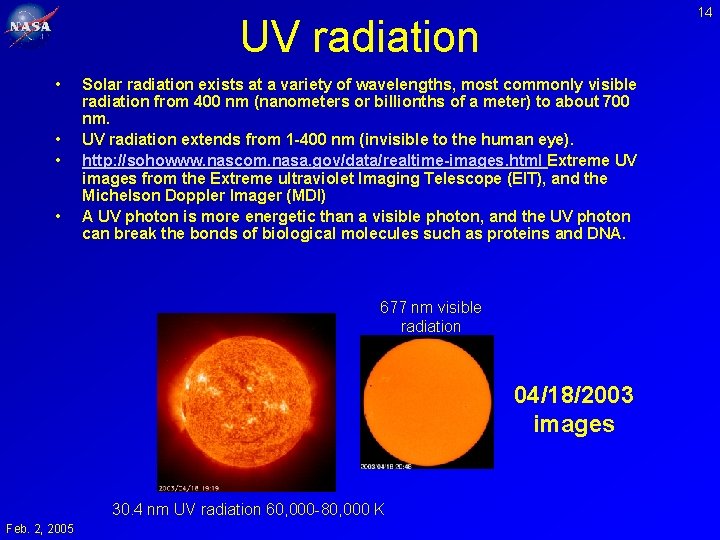
14 UV radiation • • Solar radiation exists at a variety of wavelengths, most commonly visible radiation from 400 nm (nanometers or billionths of a meter) to about 700 nm. UV radiation extends from 1 -400 nm (invisible to the human eye). http: //sohowww. nascom. nasa. gov/data/realtime-images. html Extreme UV images from the Extreme ultraviolet Imaging Telescope (EIT), and the Michelson Doppler Imager (MDI) A UV photon is more energetic than a visible photon, and the UV photon can break the bonds of biological molecules such as proteins and DNA. 677 nm visible radiation 04/18/2003 images 30. 4 nm UV radiation 60, 000 -80, 000 K Feb. 2, 2005

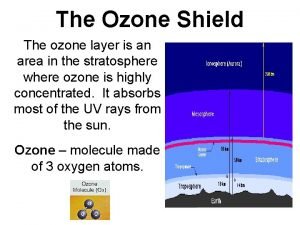
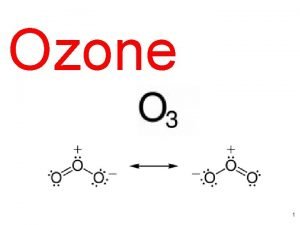
15 What is ozone? Feb. 2, 2005

16 Atmospheric Structure 100 60 Thermosphere Altitude (km) 50 Mesosphere 60 Altitude (miles) 80 40 30 40 Oxygen Ozone Stratosphere 20 20 10 Troposphere 0 Feb. 2, 2005 0

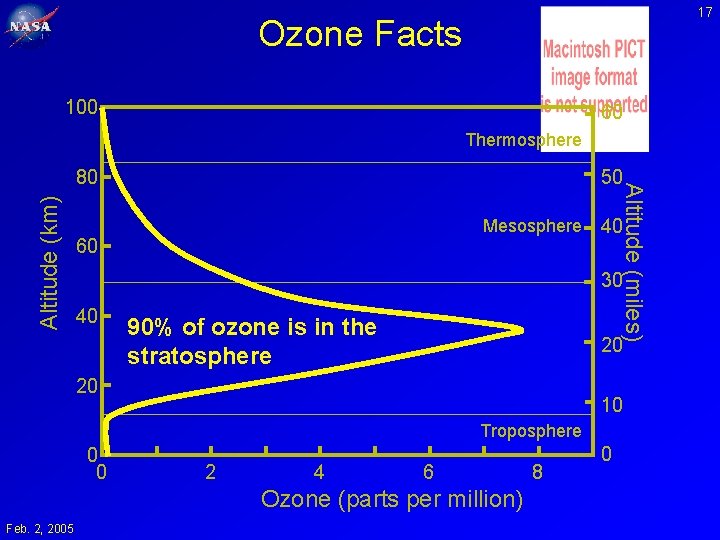
17 Ozone Facts 100 60 Thermosphere Altitude (km) 50 Mesosphere 60 Altitude (miles) 80 40 30 40 90% of ozone is in the stratosphere 20 20 10 Troposphere 0 0 2 4 6 Ozone (parts per million) Feb. 2, 2005 8 0

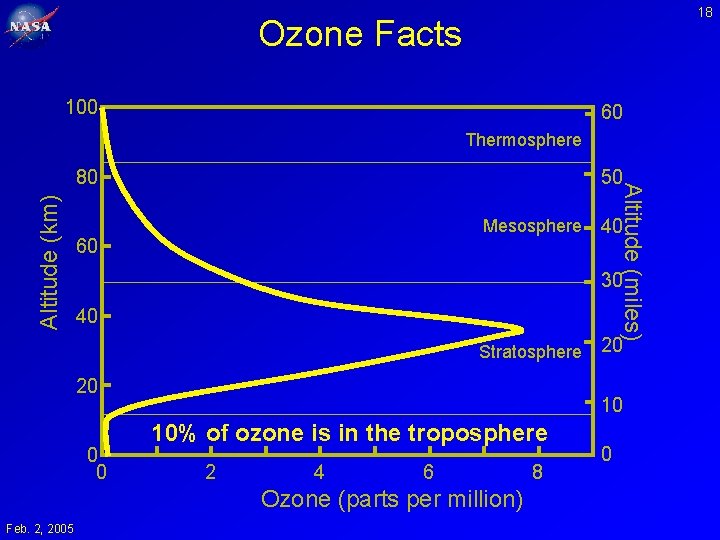
18 Ozone Facts 100 60 Thermosphere Altitude (km) 50 Mesosphere 60 40 30 40 Stratosphere 20 0 0 20 10 10% of ozone is in the troposphere 2 4 6 Ozone (parts per million) Feb. 2, 2005 Altitude (miles) 80 8 0

19 What does ozone do? Absorbs UV radiation Feb. 2, 2005

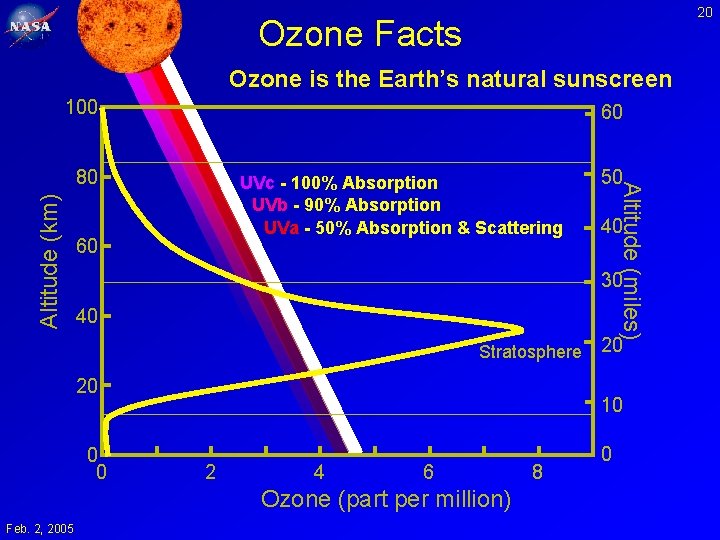
20 Ozone Facts Ozone is the Earth’s natural sunscreen 100 60 Altitude (km) UVc - 100% Absorption UVb - 90% Absorption UVa - 50% Absorption & Scattering 60 40 30 40 Stratosphere 20 0 0 20 10 2 4 6 Ozone (part per million) Feb. 2, 2005 50 Altitude (miles) 80 8 0

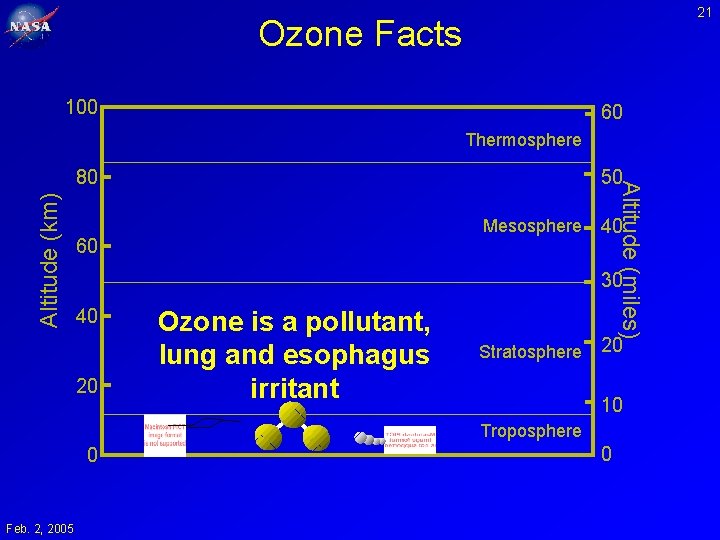
21 Ozone Facts 100 60 Thermosphere Altitude (km) 50 Mesosphere 60 Altitude (miles) 80 40 30 40 20 Ozone is a pollutant, lung and esophagus irritant Stratosphere 20 10 Troposphere 0 Feb. 2, 2005 0

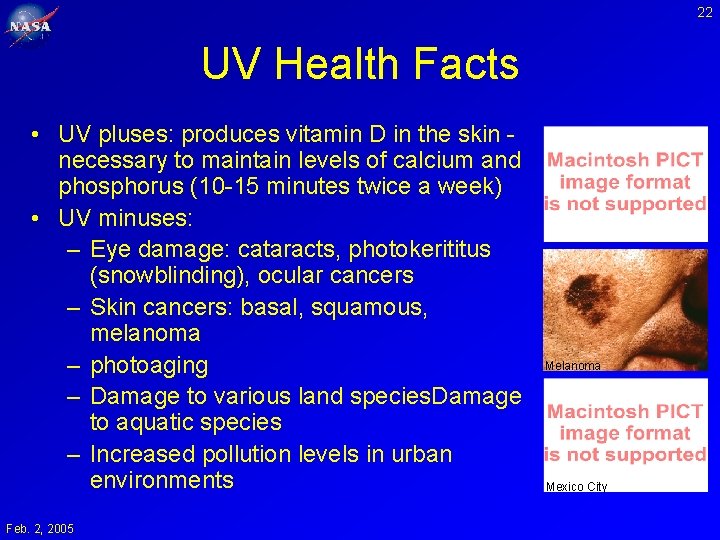
22 UV Health Facts • UV pluses: produces vitamin D in the skin necessary to maintain levels of calcium and phosphorus (10 -15 minutes twice a week) • UV minuses: – Eye damage: cataracts, photokerititus (snowblinding), ocular cancers – Skin cancers: basal, squamous, melanoma – photoaging – Damage to various land species. Damage to aquatic species – Increased pollution levels in urban environments Feb. 2, 2005 Cataract Melanoma Mexico City

25 Ozone Photochemistry Feb. 2, 2005

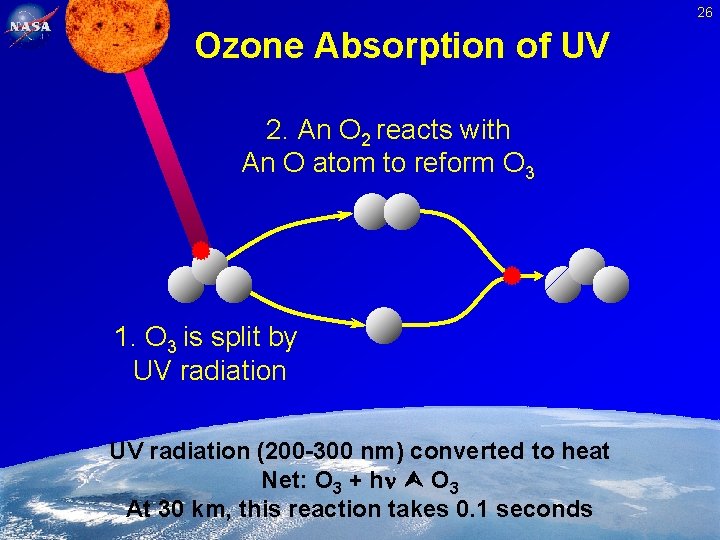
26 Ozone Absorption of UV 2. An O 2 reacts with An O atom to reform O 3 1. O 3 is split by UV radiation (200 -300 nm) converted to heat Net: O 3 + h O 3 At 30 km, this reaction takes 0. 1 seconds Feb. 2, 2005

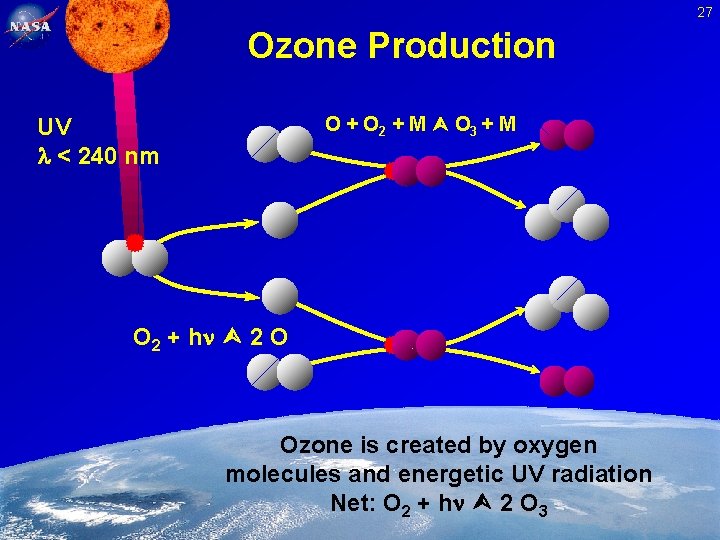
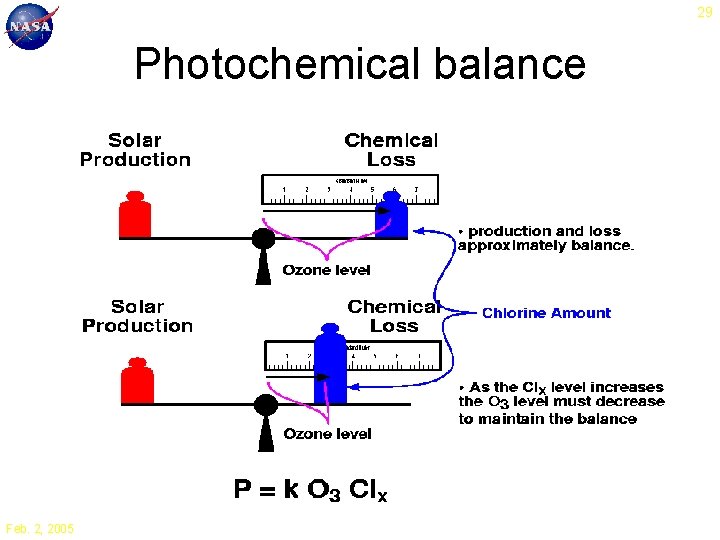
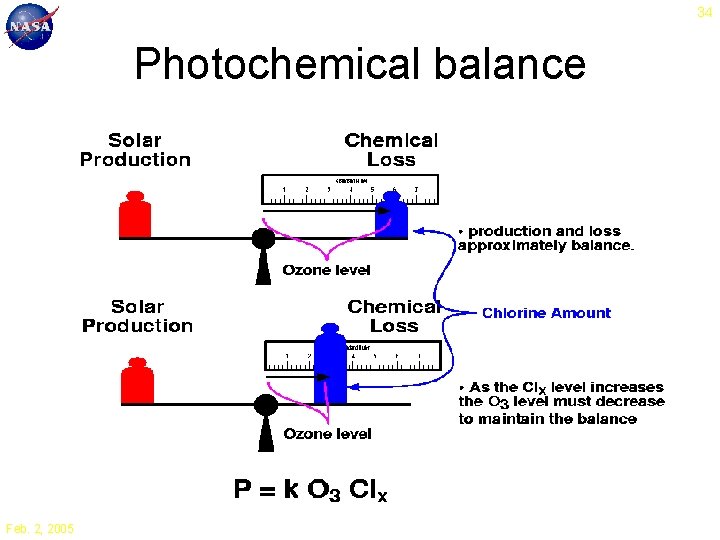
27 Ozone Production O + O 2 + M O 3 + M UV < 240 nm O 2 + h 2 O Ozone is created by oxygen molecules and energetic UV radiation Net: O 2 + h 2 O 3 Feb. 2, 2005

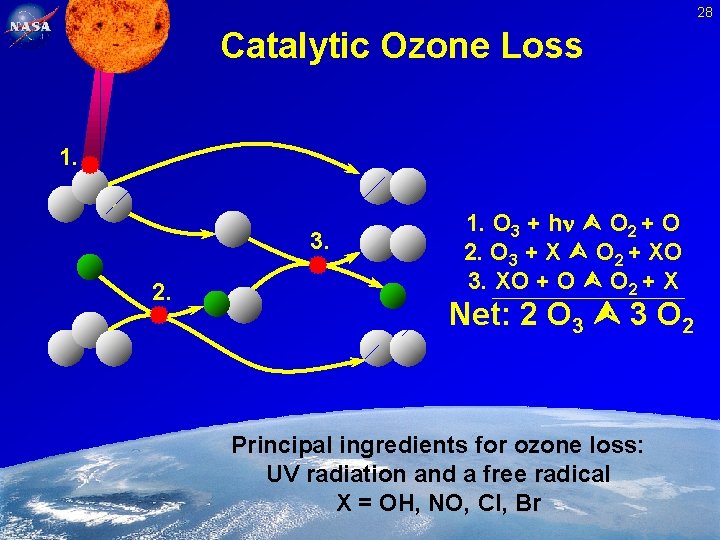
28 Catalytic Ozone Loss 1. 3. 2. 1. O 3 + h O 2 + O 2. O 3 + X O 2 + XO 3. XO + O O 2 + X Net: 2 O 3 3 O 2 Principal ingredients for ozone loss: UV radiation and a free radical X = OH, NO, Cl, Br Feb. 2, 2005

29 Photochemical balance Feb. 2, 2005

30 Source gases for ozone loss Feb. 2, 2005

32 Source Gases • Cl is much more abundant than Br • Br is about 50 times more effective at O 3 destruction Feb. 2, 2005 From Ozone FAQ - see http: //www. unep. org/ozone/faq. shtml

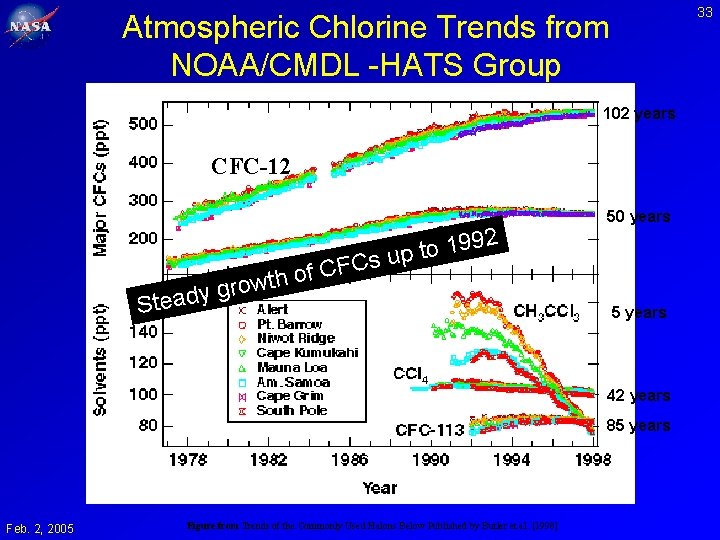
33 Atmospheric Chlorine Trends from NOAA/CMDL -HATS Group 102 years CFC-12 99 1 o t p su FC Steady f. C o h t w gro 2 50 years 5 years 42 years 85 years Feb. 2, 2005 Figure from Trends of the Commonly Used Halons Below Published by Butler et al. [1998]

34 Photochemical balance Feb. 2, 2005

36 What’s happened to polar ozone? Feb. 2, 2005

Antarctic Measurements Feb. 2, 2005 Aurora over Halley Bay Station, Antarctica, 75. 6ºS 26. 5ºE Brunt Ice Shelf, Coats Land 105 days of continuous darkness, twice per year re-supply Population: 65 in summer, 15 in winter 37

38 Digression: Dobson Units • Total Ozone is a measure of the total column amount above us. Measured in Dobson Units • If we bring all of the ozone above us down to the Earth’s surface • The thickness would be about 3 millimeters (~0. 1 inches) = 300 Dobson Units (approximately the global average) • 100 Dobson Units = 1 millimeter in thickness 2¢ 10¢ 3 mm = 300 Dobson Units Feb. 2, 2005 * The Dobson Unit is a convenient unit of measurement for total column ozone

39 October Antarctic Ozone Feb. 2, 2005 l Halley Bay October Averages Minimum value of October TOMS average

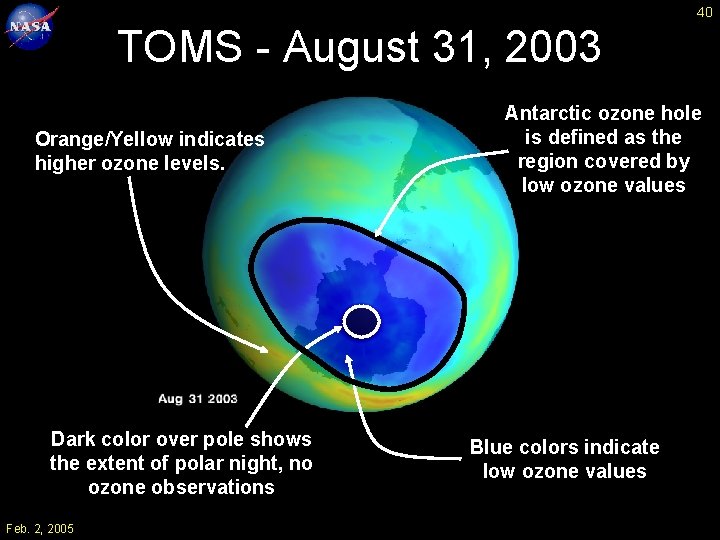
40 TOMS - August 31, 2003 Orange/Yellow indicates higher ozone levels. Dark color over pole shows the extent of polar night, no ozone observations Feb. 2, 2005 Antarctic ozone hole is defined as the region covered by low ozone values Blue colors indicate low ozone values

41 The 2003 Movie Feb. 2, 2005 Greg Shirah, NASA/GSFC SVS

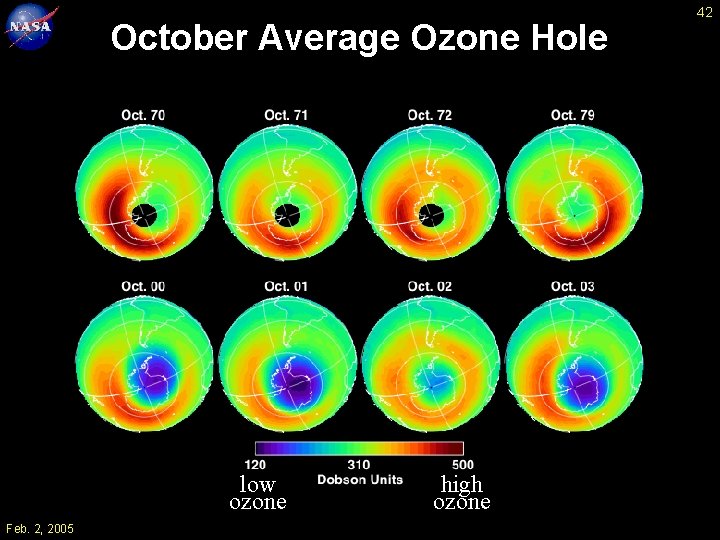
October Average Ozone Hole low ozone Feb. 2, 2005 high ozone 42

43 Arctic & Antarctic Trends Feb. 2, 2005

44 Polar Stratospheric Clouds HCl and Cl. ONO 2 react on the surface of cloud particles, releasing Cl 2. As the sun rises in the spring, the Cl 2 is photolyzed by visible light, starting a catalytic reaction that depletes ozone 1 -2% per day! Central, Sweden January 14, 2003 - P. Newman Feb. 2, 2005

Global Total Ozone Pinatubo El Chichon 60˚S-60˚N Solar Volcanoes maxima Quasi-biennial Oscillation Feb. 2, 2005 45

47 What’s being done? Feb. 2, 2005

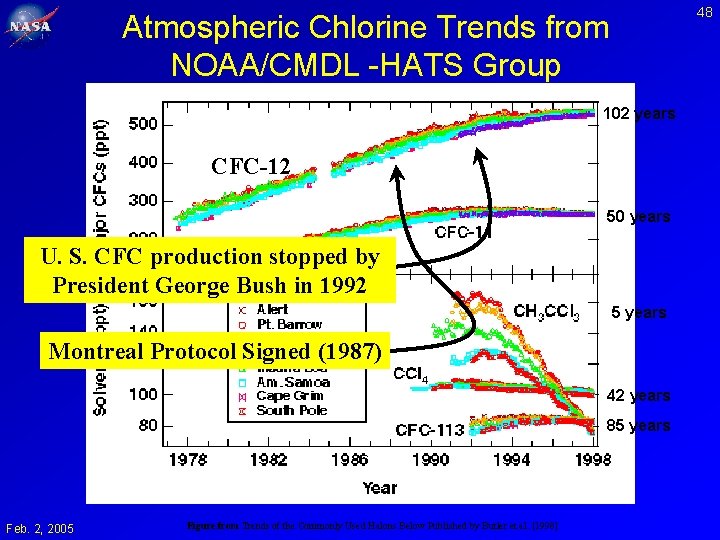
48 Atmospheric Chlorine Trends from NOAA/CMDL -HATS Group 102 years CFC-12 50 years U. S. CFC production stopped by President George Bush in 1992 5 years Montreal Protocol Signed (1987) 42 years 85 years Feb. 2, 2005 Figure from Trends of the Commonly Used Halons Below Published by Butler et al. [1998]

49 NASA continues to measure ozone and gases that destroy ozone Feb. 2, 2005

Future Antarctic Ozone Levels 3. Stratospheric water vapor (of 1. Will 2. Greenhouse CFCs and gas halons warming decrease ofincreases theaslower expected? unknown origin) increase O 3 loss atmosphere has awill cooling affectpolar in the stratosphere, increasing polar O 3 loss WMO Assessment [2003] Feb. 2, 2005 50

51 What Can You Do? • Avoid excessive solar exposure (limit sun between 11 AM and 2 PM). • Wear and encourage others to wear sunscreen (SPF rating of 15). Even with sunscreen, prolonged exposure is not smart. • Check your skin regularly. • Wear sunglasses that screen UV. • Hats and other coverings • Make note of the UV index on the news or web: http: //www. epa. gov/sunwise/uvindex. html Feb. 2, 2005

52 Summary • Stratospheric ozone is a critical gas for screening solar UV radiation. • Human produced ozone destroying substances (ODS) have caused large losses of ozone over both poles and small global losses. • ODSs have been regulated under international agreements and are slowly decreasing. Ozone levels should recover within the next 50 -70 years. Feb. 2, 2005

53 Educational Activities and Resources • Jeannie Allen (Sr. Science Education Specialist) Jeannette_Allen@ssaihq. com 301614 -6627 • See handout prepared by Jeannie • Chem Matters http: //chemistry. org/education/chemmatters. html Feb. 2, 2005

54 END Feb. 2, 2005 Jan. 10, 2003 - local noon, Kiruna, Sweden

58 What About Global Warming? Feb. 2, 2005

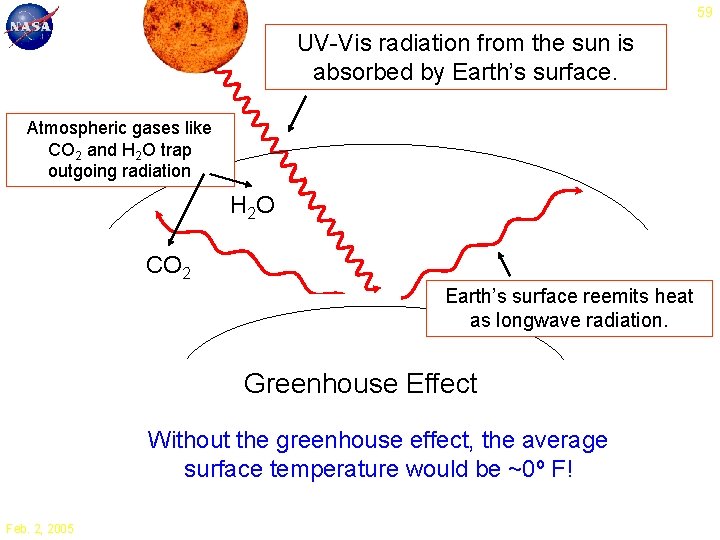
59 UV-Vis radiation from the sun is absorbed by Earth’s surface. Atmospheric gases like CO 2 and H 2 O trap outgoing radiation H 2 O CO 2 Earth’s surface reemits heat as longwave radiation. Greenhouse Effect Without the greenhouse effect, the average surface temperature would be ~0º F! Feb. 2, 2005

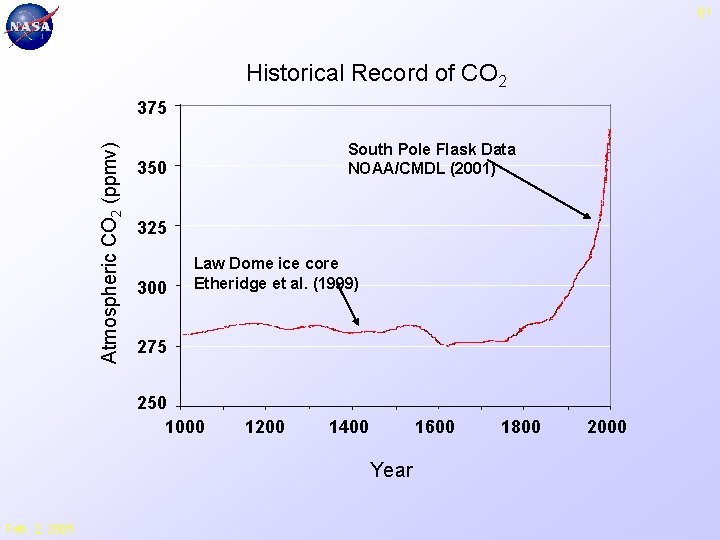
61 Historical Record of CO 2 Atmospheric CO 2 (ppmv) 375 South Pole Flask Data NOAA/CMDL (2001) 350 325 300 Law Dome ice core Etheridge et al. (1999) 275 250 1000 1200 1400 1600 Year Feb. 2, 2005 1800 2000

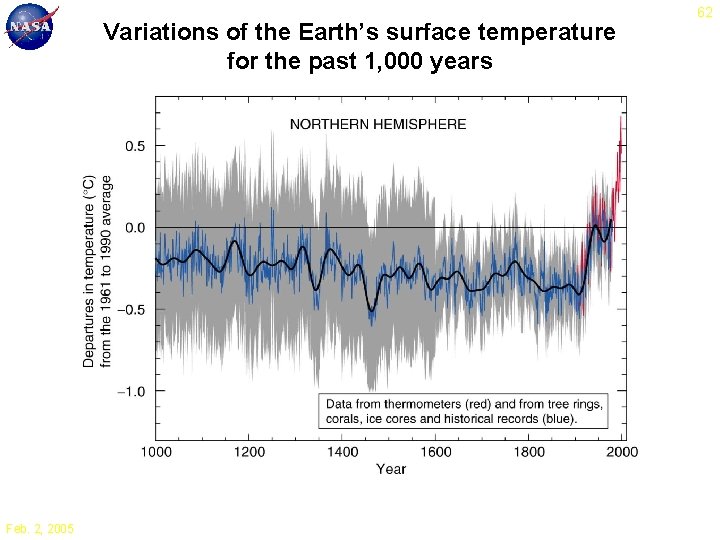
Variations of the Earth’s surface temperature for the past 1, 000 years Feb. 2, 2005 62

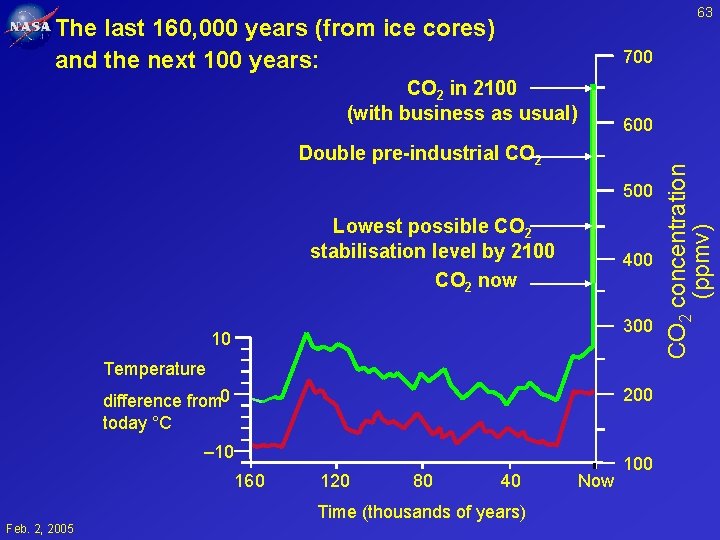
63 The last 160, 000 years (from ice cores) and the next 100 years: 700 CO 2 in 2100 (with business as usual) Double pre-industrial CO 2 500 Lowest possible CO 2 stabilisation level by 2100 CO 2 now 400 300 10 Temperature 200 difference from 0 today °C – 10 160 120 80 40 Time (thousands of years) Feb. 2, 2005 Now 100 CO 2 concentration (ppmv) 600

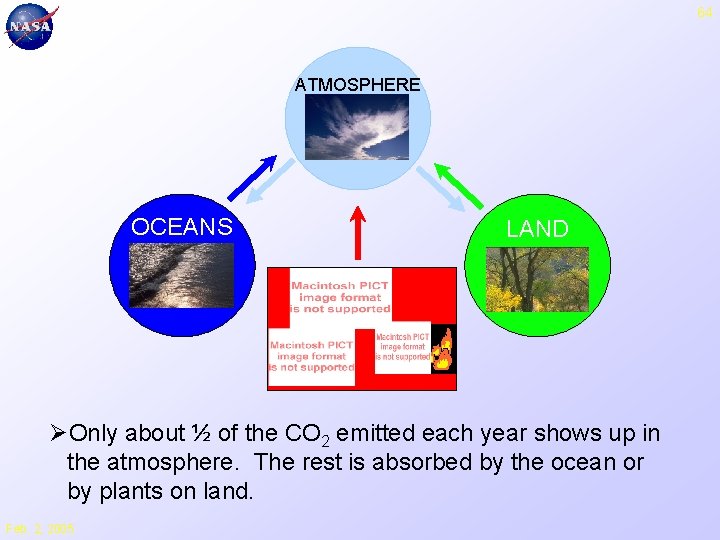
64 ATMOSPHERE OCEANS LAND ØOnly about ½ of the CO 2 emitted each year shows up in the atmosphere. The rest is absorbed by the ocean or by plants on land. Feb. 2, 2005

65 Feedbacks make predicting future climate challenging! Positive Feedback: More CO 2 Oceans Warm More H 2 O More Warming Negative Feedback: More CO 2 Oceans Warm More H 2 O More High Clouds More Reflected Sunlight Cooling Feb. 2, 2005

84 Ozone Hole Theory Feb. 2, 2005

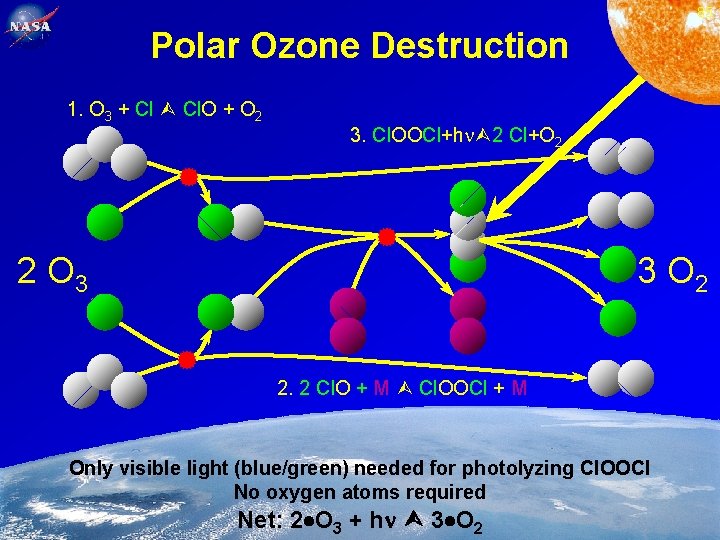
85 Polar Ozone Destruction 1. O 3 + Cl Cl. O + O 2 3. Cl. OOCl+h 2 Cl+O 2 2 O 3 3 O 2 2. 2 Cl. O + M Cl. OOCl + M Only visible light (blue/green) needed for photolyzing Cl. OOCl No oxygen atoms required Feb. 2, 2005 Net: 2 O 3 + h 3 O 2

86 Polar Processes Polar ozone losses differ from the standard photochemical balance: 1. Ozone usually has a very long lifetime: months to years 2. Ozone production is zero, losses are not compensated by production 3. Very cold conditions, cold enough to form clouds in the dry stratosphere. Feb. 2, 2005

87 Polar Stratospheric Clouds Central, Sweden January 14, 2003 - P. Newman Feb. 2, 2005

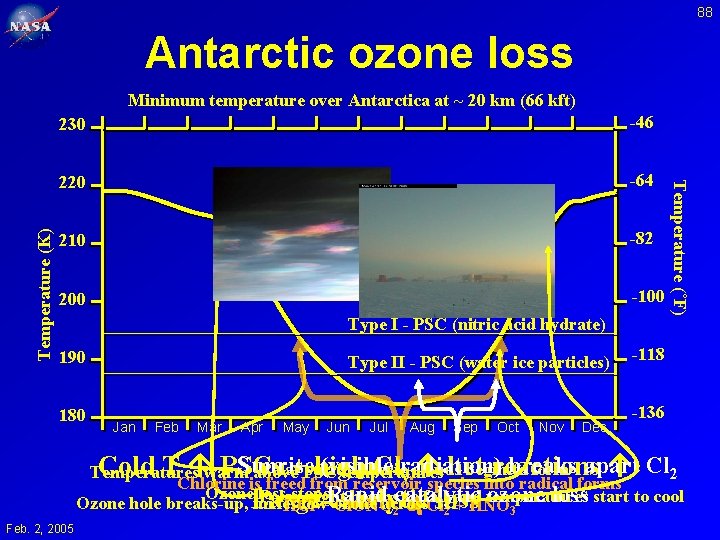
88 Antarctic ozone loss 230 -46 220 -64 210 -82 200 -100 Type I - PSC (nitric acid hydrate) 190 180 Type II - PSC (water ice particles) Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Temperature (˚F) Temperature (K) Minimum temperature over Antarctica at ~ 20 km (66 kft) -118 -136 Sunrise (visible breaks Cold T warm PSCs +PSC high Clradiation) het reactions Cl 2 Polar stratospheric clouds begin to form apart Temperatures above temperatures y Chlorine is freed from reservoir species into radical forms Ozone loss stops catalytic ozone loss start to cool Polar night begins to fall and temperatures catalytic loss Ozone hole breaks-up, Large mixes low ozone across SH HCl +Rapid Cl. ONO 2 Cl 2 + HNO 3 Feb. 2, 2005

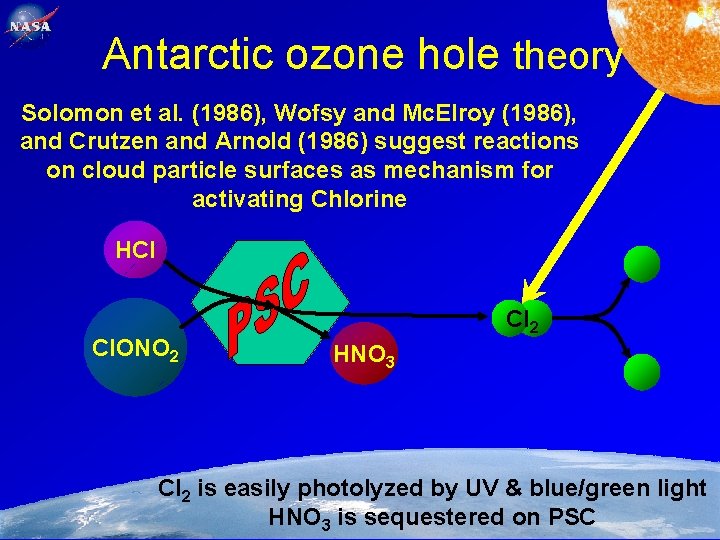
89 Antarctic ozone hole theory Solomon et al. (1986), Wofsy and Mc. Elroy (1986), and Crutzen and Arnold (1986) suggest reactions on cloud particle surfaces as mechanism for activating Chlorine HCl Cl. ONO 2 Feb. 2, 2005 Cl 2 HNO 3 Cl 2 is easily photolyzed by UV & blue/green light HNO 3 is sequestered on PSC
 Ozone depletion negative effects
Ozone depletion negative effects How do cfcs destroy ozone
How do cfcs destroy ozone Protective ozone layer
Protective ozone layer Ozone composition
Ozone composition Sopmed training
Sopmed training Microplasma ozone
Microplasma ozone Protective ozone layer
Protective ozone layer Ozone layer depletion
Ozone layer depletion Ozone layer facts
Ozone layer facts The ozone blanket blocks
The ozone blanket blocks Lewis dot shapes
Lewis dot shapes Chemical security awareness training
Chemical security awareness training Ozone layer depletion effects on humans
Ozone layer depletion effects on humans Nh4 + molecular geometry
Nh4 + molecular geometry Ncnh- lewis structure
Ncnh- lewis structure Causes of the ozone depletion
Causes of the ozone depletion Ozone layer depletion introduction
Ozone layer depletion introduction Technic
Technic Stratospheric ozone depletion
Stratospheric ozone depletion Trou d'ozone
Trou d'ozone How is total ozone distributed over the globe
How is total ozone distributed over the globe Jamoytius
Jamoytius Ozone layer made up of
Ozone layer made up of Protection of ozone layer
Protection of ozone layer Vray sun turbidity
Vray sun turbidity Ozone depletion diagram
Ozone depletion diagram Global warming vs ozone depletion
Global warming vs ozone depletion Ozone without borders
Ozone without borders Ozone hole myth
Ozone hole myth Ozone layer levels
Ozone layer levels Benjamin cummings
Benjamin cummings Ozone nasa
Ozone nasa The ozone layer protects us from
The ozone layer protects us from Ground level ozone effects
Ground level ozone effects Solid state marx generator
Solid state marx generator Ozone layer
Ozone layer Where are the holes in the ozone layer located
Where are the holes in the ozone layer located Wheres the ozone layer
Wheres the ozone layer Ozone klavye
Ozone klavye How is smog formed
How is smog formed Atmospheric opacity
Atmospheric opacity Atmospheric perspective watercolor
Atmospheric perspective watercolor Atmospheric suits
Atmospheric suits Atmospheric gravity waves
Atmospheric gravity waves Penn state meteorology jobs
Penn state meteorology jobs Lab 5 atmospheric moisture
Lab 5 atmospheric moisture Atmospheric pressure at different altitudes
Atmospheric pressure at different altitudes Forceparcel
Forceparcel Single cell model of atmospheric circulation
Single cell model of atmospheric circulation Atmospheric stability
Atmospheric stability Atmospheric stability
Atmospheric stability Conditionally unstable atmosphere
Conditionally unstable atmosphere Single cell model of atmospheric circulation
Single cell model of atmospheric circulation Atmospheric heaven
Atmospheric heaven The four main layers of the atmosphere
The four main layers of the atmosphere Atmospheric opacity
Atmospheric opacity Atmospheric circulation
Atmospheric circulation Atmospheric distortion correction
Atmospheric distortion correction What is weather variables
What is weather variables Earths layers definition
Earths layers definition Apes unit 2
Apes unit 2 A first course in atmospheric thermodynamics solutions
A first course in atmospheric thermodynamics solutions Atmospheric convection
Atmospheric convection