ATMOSPHERIC STRUCTURE AND COMPOSITION THE ATMOSPHERE Atmosphere the


































- Slides: 43

ATMOSPHERIC STRUCTURE AND COMPOSITION

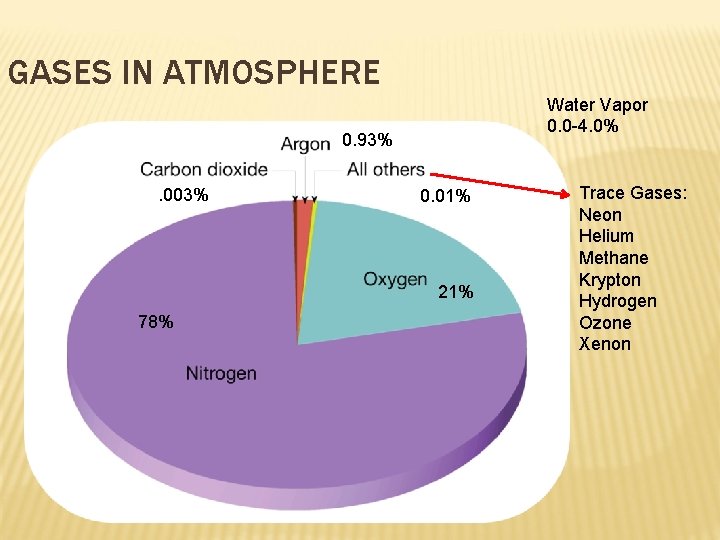
THE ATMOSPHERE Atmosphere = the thin layer of gases around Earth Provides oxygen Absorbs radiation and moderates climate Transports and recycles water and nutrients 78% N 2, 21% O 2 Density of air decreases with increasing altitude (height above Earth’s surface) Air: A mechanical mixture of gases and aerosols, very small solid particles or liquid droplets suspended in the atmosphere Minute (my nyoot…very small) concentrations of permanent gases (remain at stable concentrations) Variable gases = varying concentrations across time and place Human activity is changing the amount of some gases:

THE FORMATION OF OUR ATMOSPHERE!

STEP 1: SUPERNOVA EXPLOSION – FIRST ATMOSPHERE MADE OF H 2 AND O 2 The formation of Earth with an atmosphere made of hydrogen and oxygen. http: //www. mydigitallife. info/wp-content/uploads/2007/05/supernova_sn 2006 gy. jpg

STEP 2: SOLAR WIND BLEW AWAY FIRST ATMOSPHERE Was a magnetic field present? http: //astroprofspage. com/wpcontent/uploads/2006/11/magneto. jpg

STEP 3: VOLCANIC ERUPTIONS RESULT IN… Solid surface of the earth was formed and many gases such as nitrogen, carbon dioxide, water vapor, and sulfur dioxide are released forming a second atmosphere. http: //cache. virtualtourist. com/1719818 Volcanic_eruption_in_Vatnajokul_glacier-Iceland. jpg

STEP 4 - COOLING OF THE EARTH’S SURFACE AND THE ATMOSPHERE It rains and rains.

STEP 5 -LIGHTNING AND TORRENTIAL STORMS RESULTS IN… Lightning breaks apart water molecules in the air, creating free oxygen which helps to form ozone.

STEP 6 - PRESENCE OF PHOTOSYNTHESIZING ORGANISMS RESULTS IN… Simple aquatic plants took in CO 2 and released O 2 to atmosphere via photosynthesis Carbon dioxide in the atmosphere decreases and oxygen levels

COMPOSITION OF THE ATMOSPHERE:

GASES IN ATMOSPHERE Water Vapor 0. 0 -4. 0% 0. 93%. 003% 0. 01% 21% 78% Trace Gases: Neon Helium Methane Krypton Hydrogen Ozone Xenon

WHY ARE TRACE GASES IMPORTANT H 2 O Vapor, CO 2, Methane, Ozone Varies with season, altitude of particular air mass, and surface features beneath the air (ex. Air over desert is drier than over ocean) Source of clouds, rain and snow Regulates amount of energy the atmosphere absorbs Regulates temperature, source of precipitation, helps create weather

WHY ARE TRACE GASES IMPORTANT? Carbon Dioxide- regulates heat in the atmosphere (Green house gas), Amplifies heating of atmosphere, plants need it for photosynthesis. Ozone (O 3), - form of oxygen that blocks UV rays

How is Ozone made? 1. sunlight splits an O 2 molecule, forming 2 single O molecules 2. One new O atom collides with an O 2 molecule 3. New O 3 molecule formed

AEROSOLS: SOLIDS IN THE ATMOSPHERE Salt Picked up from ocean spray Aids in cloud formation Dust Carried by the wind Aids in cloud formation Ice Particulates- ash, soot, solids from burning fuels

17. 1 ATMOSPHERE CHARACTERISTICS Composition of the Atmosphere Human Influence • Emissions from transportation vehicles account for nearly half the primary pollutants by weight.

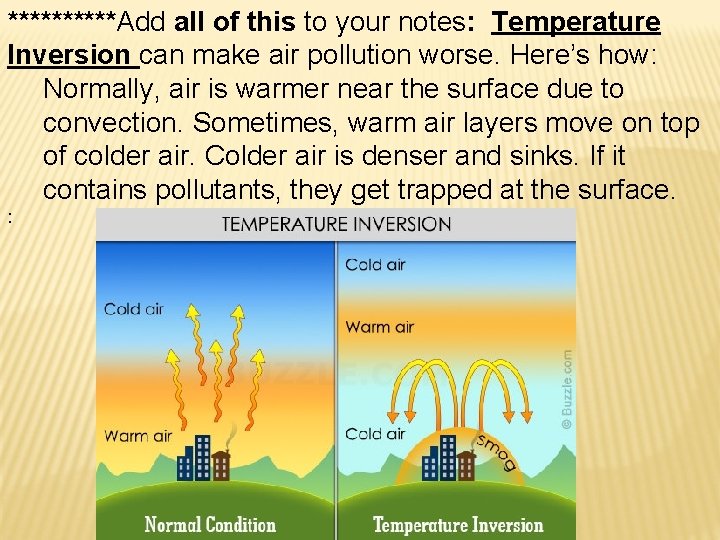
AIR POLLUTION Any substance in the atmosphere that is harmful Sources include: Burning fossil fuels – coal and petroleum Temperature next slide… Inversion -Important info on the

*****Add all of this to your notes: Temperature Inversion can make air pollution worse. Here’s how: Normally, air is warmer near the surface due to convection. Sometimes, warm air layers move on top of colder air. Colder air is denser and sinks. If it contains pollutants, they get trapped at the surface. :

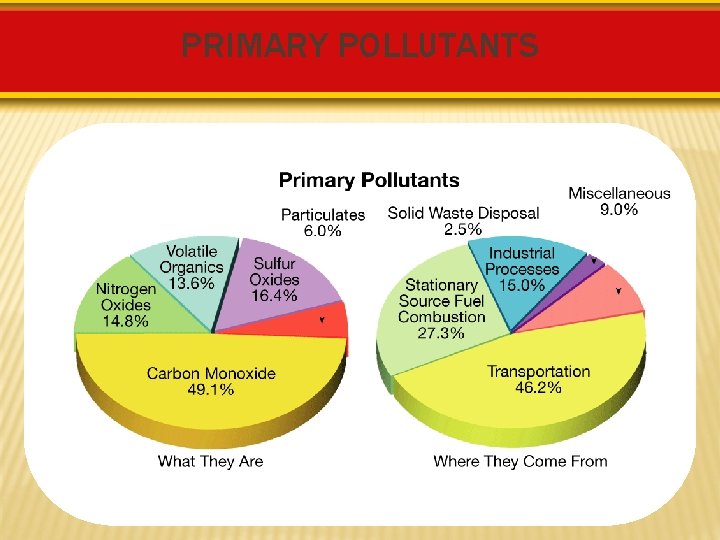
PRIMARY POLLUTANTS

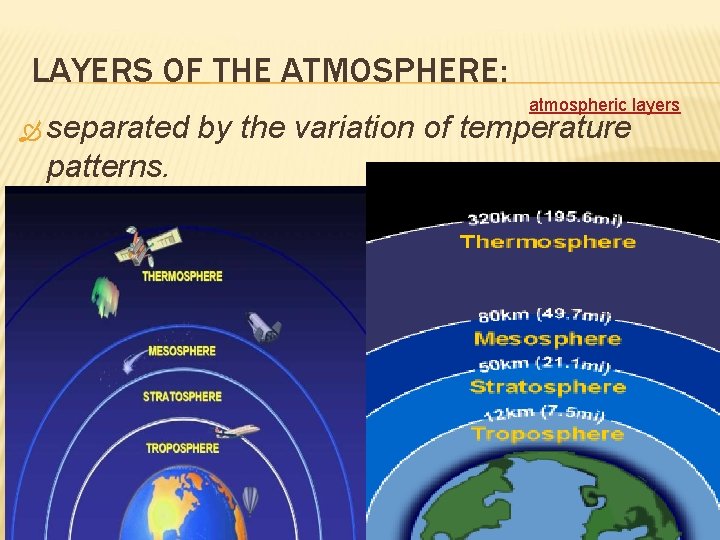
LAYERS OF THE ATMOSPHERE: separated patterns. atmospheric layers by the variation of temperature

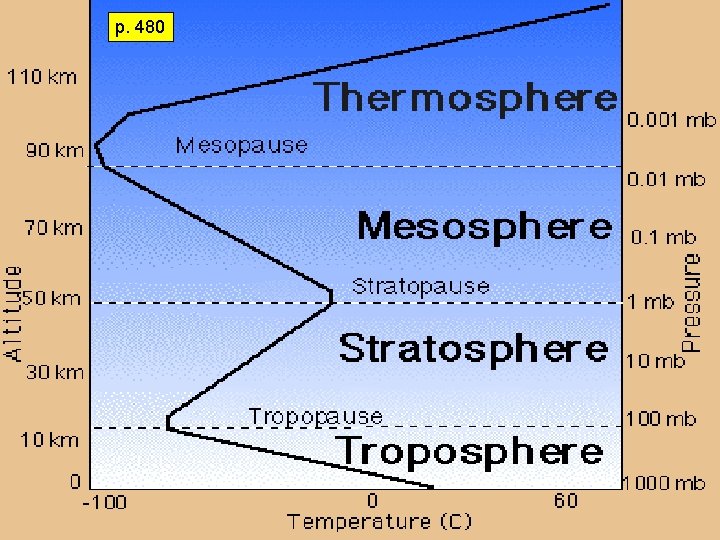
p. 480


Pressure & as the. Temperature altitude increases, air pressure decreases This is called an INVERSE relationship

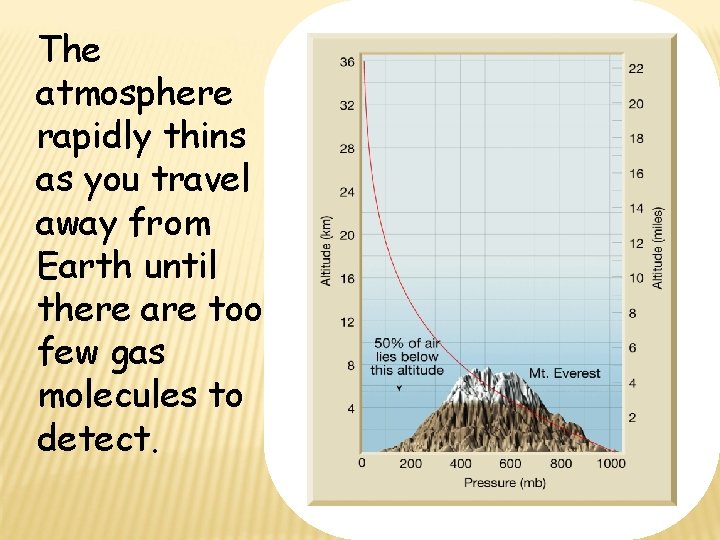
The atmosphere rapidly thins as you travel away from Earth until there are too few gas molecules to detect.

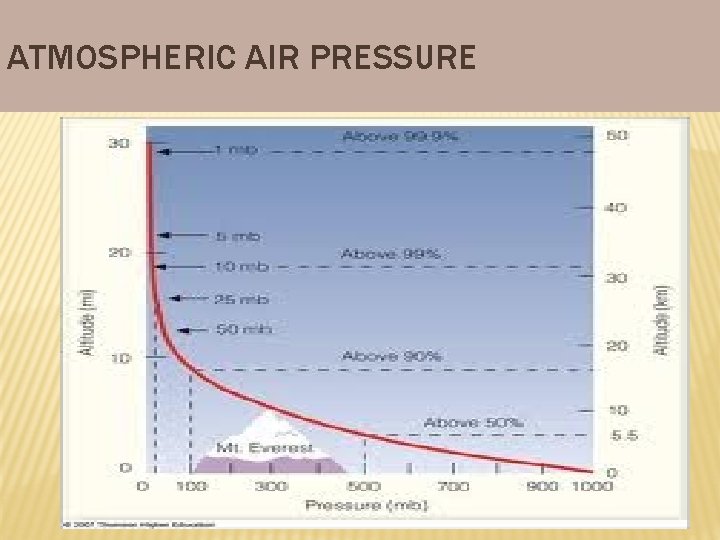
ATMOSPHERIC PRESSURE • • Atmospheric Pressure Air pressure is the force air exerts per unit area. Air pressure is measured with a barometer. Air pressure decreases as altitude increases. You do notice the pressure because you have always lived with it.

ATMOSPHERIC AIR PRESSURE

Atmospheric Layers there are 4 layers based on temperature changes. Troposphere Is this something I should remember? YES!!! Stratosphere Mesosphere Thermosphere

TROPOSPHERE Layer nearest Earth 0 -16 km We live in the troposphere. All weather and water vapor are in this layer. Clouds form in this layer Gets colder as you go up. Airplanes Fly Temperature drops as altitude increases, Density is greatest in this layer of the atmosphere (gravity is stronger so air is denser – air molecules tightly packed) Contains over 80% of the atmosphere More than 50% of the atmosphere is located below 5. 6 km “The Troublesphere”

STRATOSPHERE 16– 48 km (7– 31 mi) above sea level Temperature stays constant, then starts to increase as altitude increases This is because there is a lot of ozone in the stratosphere and ozone absorbs heat. Home of the Ozone layer is not constant. Changes began to be noticed in the ozone above Antarctica in the 1970’s. This is the layer that absorbs ultraviolet radiation and it becomes heated. Planes will fly in lowest part of this layer to avoid storms. This is warmer than the troposphere. Drier and less dense, with little vertical mixing The troposphere and stratosphere together make up the lower atmosphere 99% of all atmospheric air is found in the lower atmosphere


OZONE LAYER Lower portion of stratosphere From about 12 to 19 miles up. Varies seasonally and geographically High concentration of ozone

READ EVERY SLIDE CAREFULLY!

OZONE LAYER Ozone (O 3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's lower stratosphere (natural ozone) and near the surface in the troposphere (ground-level ozone). Depending on where it is in the atmosphere, ozone affects life on Earth in either good or bad ways.

OZONE LAYER The ozone that is commonly associated with ozone depletion and the ozone “hole” is in the stratosphere, a layer that extends upward from about 10 to 30 miles (15 to 50 kilometers) above the earth. This “good” ozone shields life on earth from the sun's harmful ultraviolet rays (UV-b) by absorbing them. The layer surrounding the earth's surface is the troposphere. Here, ground-level or "bad" ozone is an air pollutant that damages human health, vegetation, and many common materials. It is a key ingredient of urban smog. The troposphere extends from the surface to about 10 miles (16 kilometers) above the surface. Ground level, or “bad ozone”, is also called tropospheric ozone while “good ozone” is called stratospheric ozone. About 80 -90% of all ozone in the atmosphere is found in the stratosphere.

OZONE LAYER Chlorofluorocarbons (CFCs), chemicals found mainly in spray aerosols heavily used by industrialized nations for much of the past 50 years, are the primary culprits in ozone layer breakdown In 1978, the United States, Canada and Norway enacted bans on CFC-containing aerosol sprays that damage the ozone layer. On August 2, 2003, scientists announced that the global depletion of the ozone layer may be slowing down due to the international regulation of ozonedepleting substances.

MESOSPHERE 48– 80 km (31– 56 mi) above sea level Temperature decreases as altitude increases. Extremely low air pressure Coldest layer of the atmosphere Radio waves are reflected to Earth. Meteors burn up in this layer.

THERMOSPHERE Extends upward from about 80 km to 500 -600 km (300 mi) Temperature increases as altitude increases due to the absorption of very short-wave solar energy by oxygen. It is the hottest layer Contains electrically charged particles that cause Aurora lights Satellites orbit space in this layer of Earth’s atmosphere. Ionosphere is the lower region which contains ions and reflects radio waves back to earth Exosphere is where the earth’s atmosphere blends


IMPORTANCE OF THE ATMOSPHERE Earth’s atmosphere makes conditions on Earth suitable for living things. The atmosphere is constantly changing as the gases move in and out of living things, the land, and the water. It works to trap the sun’s energy, move the heat around, and make it possible for life to exist.

IMPORTANCE OF THE ATMOSPHERE Earth’s atmosphere traps energy from the sun, which allows water to exist in a liquid form. Allowing water to exist in a liquid form allows for life to happen, as water is essential for living things.

KEEP READING. FINISH THE POWERPOINT! ONLY 2 MORE SLIDES.

Almost all of the Earth’s energy comes from the sun and is called radiant energy. • most reaches the atmosphere and is reflected back to space

• Some is absorbed by the Earth and is spread throughout the atmosphere as: • Radiation: transfer of energy in the form of waves • Unlike conduction and convection, which need material to travel through, radiant energy can travel through the vacuum of space. This leads into the next PPt, “Heating of the Atmosphere”.