The Earth and Its Atmosphere Chemical composition and



- Slides: 18

The Earth and Its Atmosphere: Chemical composition and vertical structure

• • • RECAP Definition of an atmosphere: the gas surrounding a planet/satellite/comet/… Origin of the atmosphere. Three stages: ♦ I - gravitational capture of the gasses in the proto planetary nebula of the Sun (mainly H 2, He); ♦ II - outgassing of the planet (volcanoes, geysers, …); formation of an ocean (perhaps? ); material from meteorites and comets; ♦ III – evolution of the atmosphere due to the presence of life and human activity. The early atmosphere of the Earth is very different from the atmosphere today! We learn about the formation and the evolution of the Earth’s atmosphere from the Earth’s geological records and by studying other planets. The role of the atmosphere: protection from UV and cosmic rays, shields us from meteorites, decreases the day/night temperature variations…

• • Planets in the Solar System Definition of a planet: a celestial body which ♦ is in orbit around the sun ♦ has sufficient mass for its self-gravity to overcome rigid body forces so that it assumes a. . . nearly round shape ♦ has cleared the neighborhood around its orbit Pluto disqualified since orbit overlaps with Neptune's.

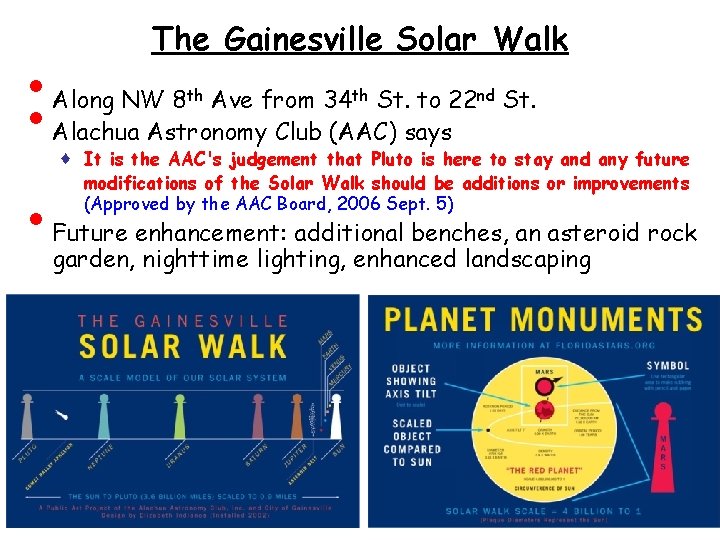
• • • The Gainesville Solar Walk Along NW 8 th Ave from 34 th St. to 22 nd St. Alachua Astronomy Club (AAC) says ♦ It is the AAC's judgement that Pluto is here to stay and any future modifications of the Solar Walk should be additions or improvements (Approved by the AAC Board, 2006 Sept. 5) Future enhancement: additional benches, an asteroid rock garden, nighttime lighting, enhanced landscaping

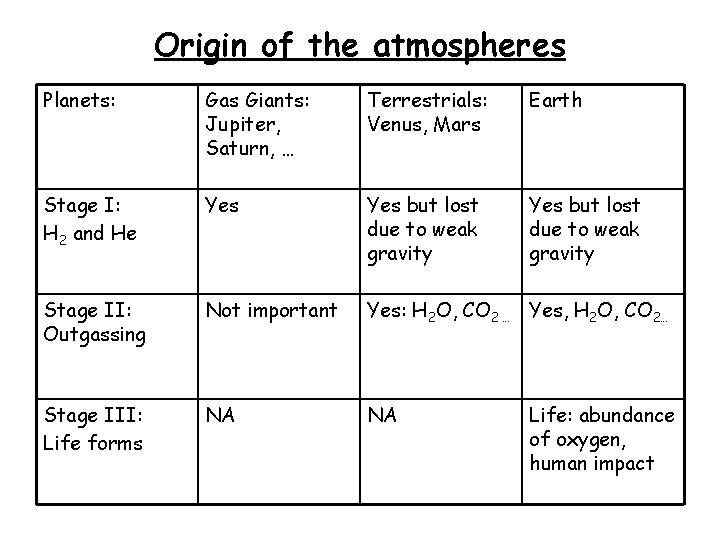
Origin of the atmospheres Planets: Gas Giants: Jupiter, Saturn, … Terrestrials: Venus, Mars Earth Stage I: H 2 and He Yes but lost due to weak gravity Stage II: Outgassing Not important Yes: H 2 O, CO 2 … Yes, H 2 O, CO 2… Stage III: Life forms NA NA Life: abundance of oxygen, human impact

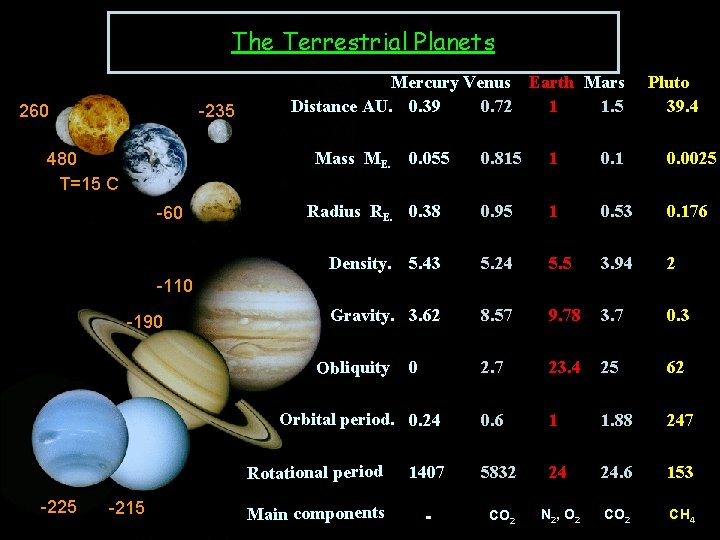
The Terrestrial Planets 260 -235 Mercury Venus Earth Mars Distance AU. 0. 39 0. 72 1 1. 5 Mass ME. 480 T=15 C -60 0. 055 Pluto 39. 4 0. 815 1 0. 0025 0. 95 1 0. 53 0. 176 5. 43 5. 24 5. 5 3. 94 2 Gravity. 3. 62 8. 57 9. 78 3. 7 0. 3 2. 7 23. 4 25 62 0. 6 1 1. 88 247 Radius RE. 0. 38 Density. -110 -190 Obliquity 0 Orbital period. 0. 24 -225 -215 Rotational period 1407 5832 24 24. 6 153 Main components - CO 2 N 2, O 2 CH 4

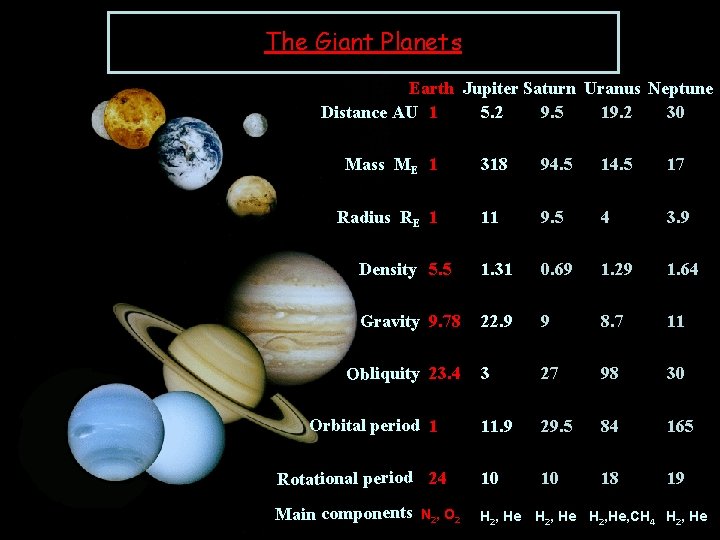
The Giant Planets Earth Jupiter Saturn Uranus Neptune Distance AU 1 5. 2 9. 5 19. 2 30 Mass ME 1 318 94. 5 17 Radius RE 1 11 9. 5 4 3. 9 Density 5. 5 1. 31 0. 69 1. 29 1. 64 Gravity 9. 78 22. 9 9 8. 7 11 3 27 98 30 11. 9 29. 5 84 165 Rotational period 24 10 10 18 19 Main components H 2, He Obliquity 23. 4 Orbital period 1 N 2, O 2 H 2, He, CH 4 H 2, He

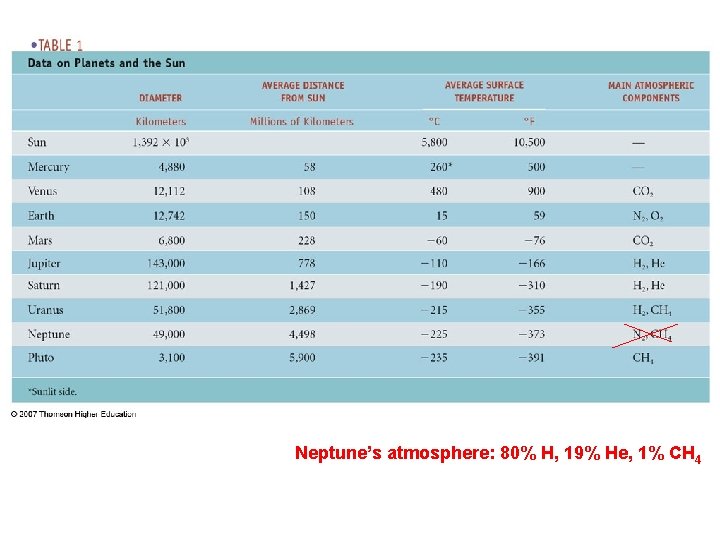
Neptune’s atmosphere: 80% H, 19% He, 1% CH 4

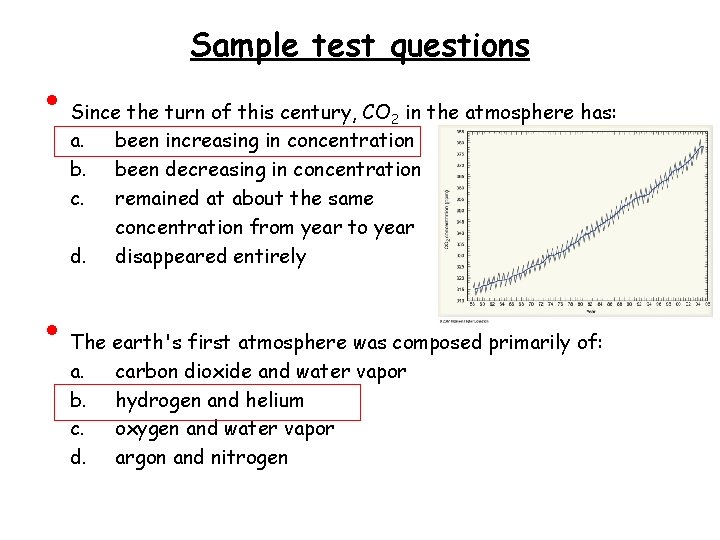
• • Sample test questions Since the turn of this century, CO 2 in the atmosphere has: a. been increasing in concentration b. been decreasing in concentration c. remained at about the same concentration from year to year d. disappeared entirely The earth's first atmosphere was composed primarily of: a. carbon dioxide and water vapor b. hydrogen and helium c. oxygen and water vapor d. argon and nitrogen

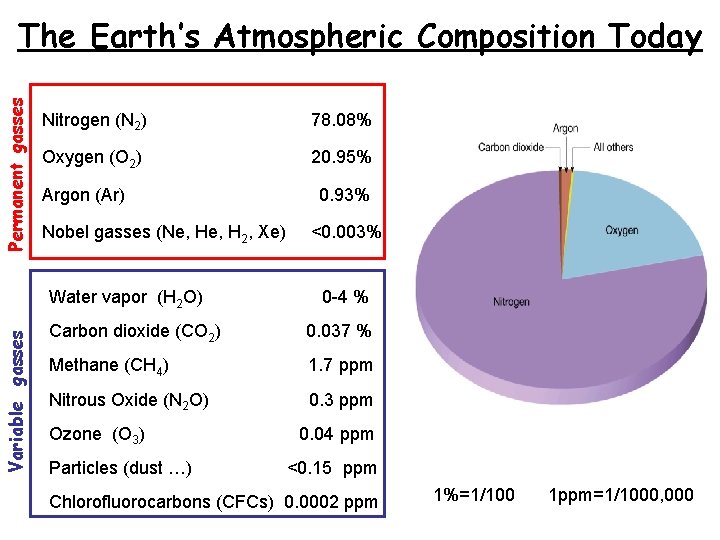
Permanent gasses The Earth’s Atmospheric Composition Today Nitrogen (N 2) 78. 08% Oxygen (O 2) 20. 95% Argon (Ar) Nobel gasses (Ne, H 2, Xe) Variable gasses Water vapor (H 2 O) 0. 93% <0. 003% 0 -4 % Carbon dioxide (CO 2) 0. 037 % Methane (CH 4) 1. 7 ppm Nitrous Oxide (N 2 O) 0. 3 ppm Ozone (O 3) Particles (dust …) 0. 04 ppm <0. 15 ppm Chlorofluorocarbons (CFCs) 0. 0002 ppm 1%=1/100 1 ppm=1/1000, 000


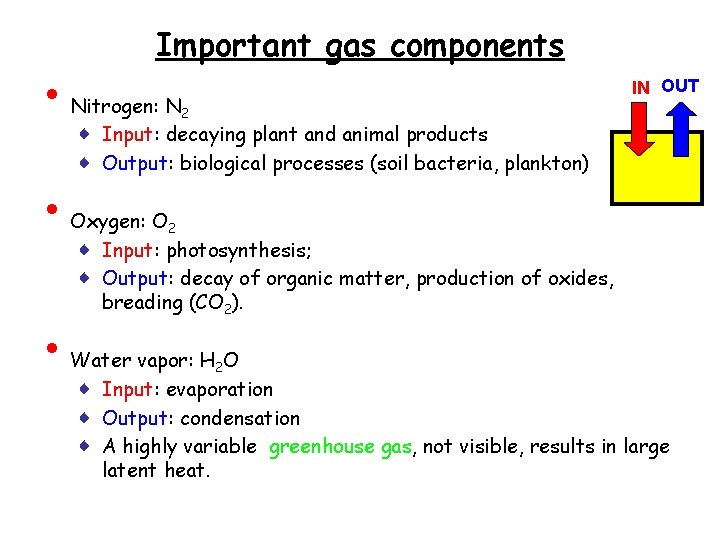
• • • Important gas components Nitrogen: N 2 ♦ Input: decaying plant and animal products ♦ Output: biological processes (soil bacteria, plankton) IN OUT Oxygen: O 2 ♦ Input: photosynthesis; ♦ Output: decay of organic matter, production of oxides, breading (CO 2). Water vapor: H 2 O ♦ Input: evaporation ♦ Output: condensation ♦ A highly variable greenhouse gas, not visible, results in large latent heat.

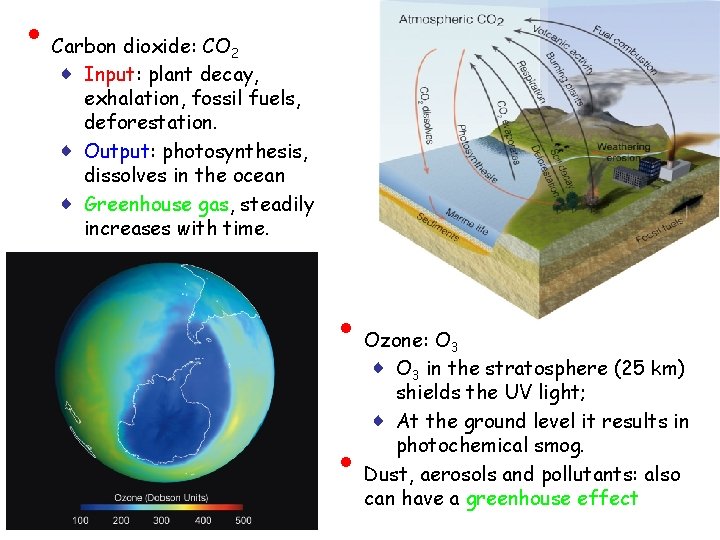
• Carbon dioxide: CO 2 ♦ Input: plant decay, exhalation, fossil fuels, deforestation. ♦ Output: photosynthesis, dissolves in the ocean ♦ Greenhouse gas, steadily increases with time. CO 2 • • Ozone: O 3 ♦ O 3 in the stratosphere (25 km) shields the UV light; ♦ At the ground level it results in photochemical smog. Dust, aerosols and pollutants: also can have a greenhouse effect

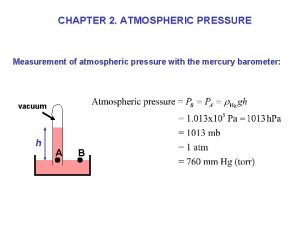
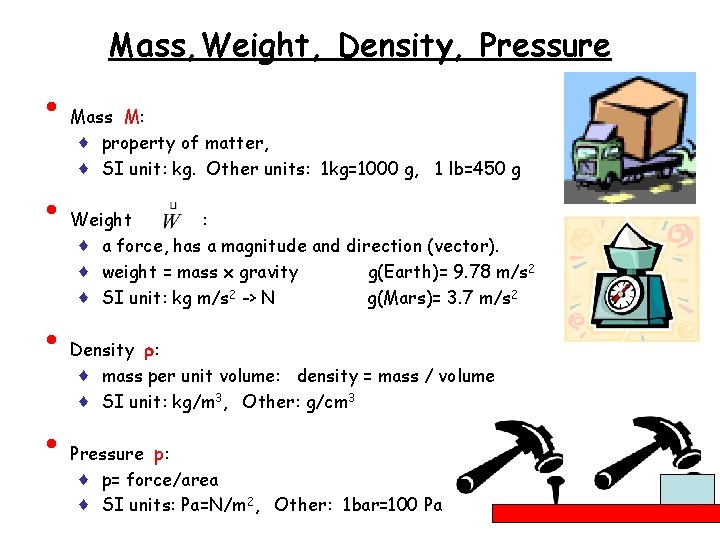
Mass, Weight, Density, Pressure • • Mass M: ♦ property of matter, ♦ SI unit: kg. Other units: 1 kg=1000 g, 1 lb=450 g Weight : ♦ a force, has a magnitude and direction (vector). ♦ weight = mass x gravity g(Earth)= 9. 78 m/s 2 ♦ SI unit: kg m/s 2 -> N g(Mars)= 3. 7 m/s 2 Density r: ♦ mass per unit volume: density = mass / volume ♦ SI unit: kg/m 3, Other: g/cm 3 Pressure p: ♦ p= force/area ♦ SI units: Pa=N/m 2, Other: 1 bar=100 Pa

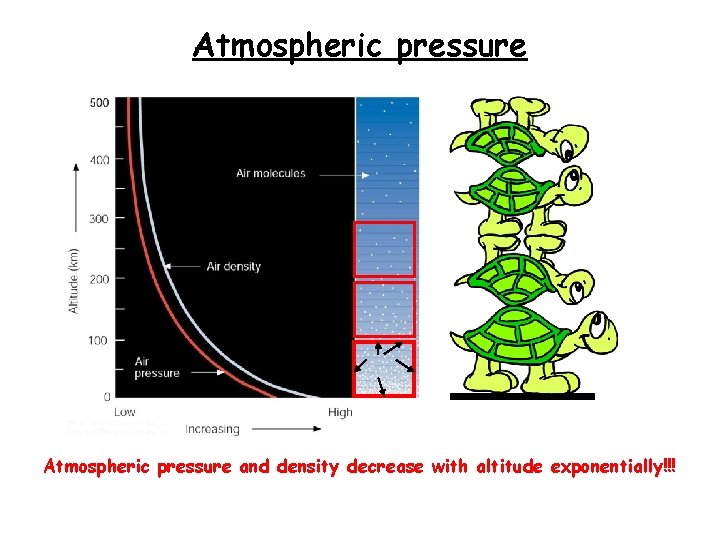
Atmospheric pressure and density decrease with altitude exponentially!!!

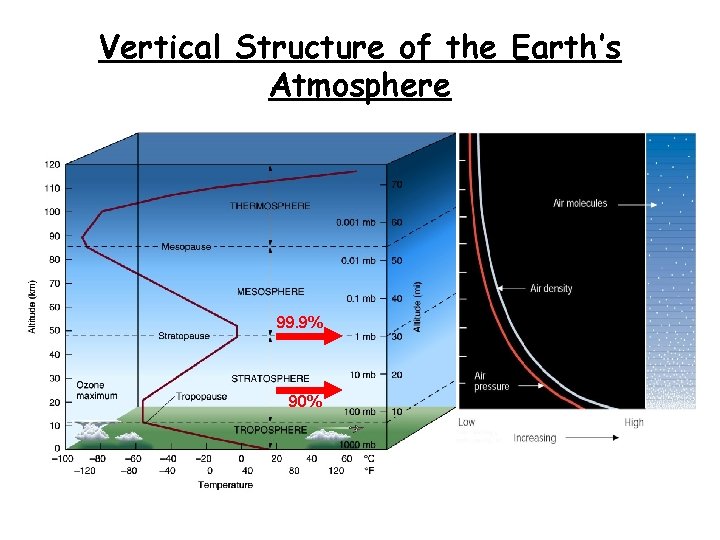
Vertical Structure of the Earth’s Atmosphere 99. 9% 90%

• • • Atmospheric layers (according to the temperature) Troposphere: ♦ The temperature T decreases with height about 6. 5 K/km. ♦ Well mixed as a result of turbulence and convection; ♦ Weather phenomena Tropopause: ♦ isothermal (T constant) ♦ located 8 -15 km above the ground. Stratosphere: ♦ Increasing temperature; ♦ O 3 layer at 25 km altitude; ♦ The atmosphere is very stable. Stratopause: T=const Mesosphere: ♦ T is decreasing: effective cooling through IR emission. Mesopause: the coldest region on Earth. Thermosphere: fast T increase. Diffusive separation of gasses.

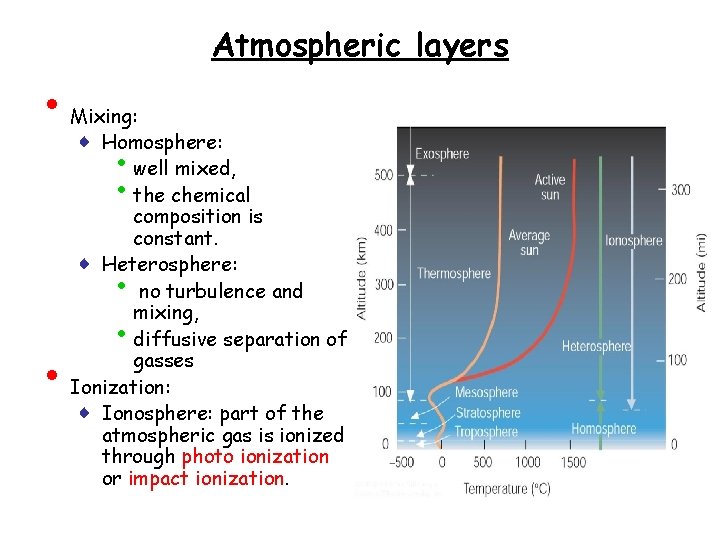
Atmospheric layers • Mixing: ♦ Homosphere: well mixed, the chemical composition is constant. ♦ Heterosphere: no turbulence and mixing, diffusive separation of gasses Ionization: ♦ Ionosphere: part of the atmospheric gas is ionized through photo ionization or impact ionization. • • •
 Structure of atmosphere diagram
Structure of atmosphere diagram Atmosphere ideas
Atmosphere ideas Hsocl
Hsocl Types of atmosphere
Types of atmosphere The atmosphere protects earth from
The atmosphere protects earth from Edible aquifer lab activity answers
Edible aquifer lab activity answers Thermosphere
Thermosphere Earth atmosphere ppt
Earth atmosphere ppt Earth atmosphere radius
Earth atmosphere radius The atmosphere protects earth from
The atmosphere protects earth from Describing earth's atmosphere lesson 1 answer key
Describing earth's atmosphere lesson 1 answer key Earth's atmosphere
Earth's atmosphere Earth's atmosphere description
Earth's atmosphere description Earth surface atmosphere
Earth surface atmosphere Earth’s atmosphere
Earth’s atmosphere Gases on earth's atmosphere
Gases on earth's atmosphere Matter and its composition
Matter and its composition Mesosphere earth layer composition
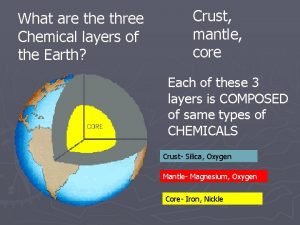
Mesosphere earth layer composition Three chemical layers of the earth
Three chemical layers of the earth