Today in I S Pick up Week 1






























- Slides: 59

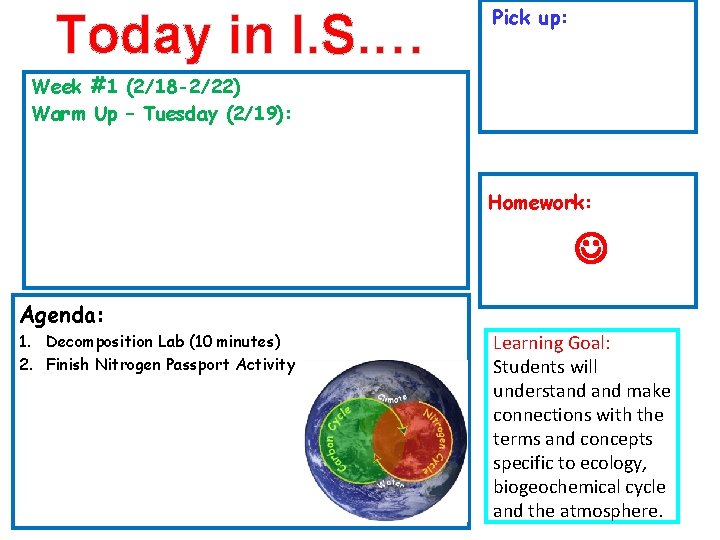
Today in I. S. … Pick up: Week #1 (2/18 -2/22) Warm Up – Tuesday (2/19): Homework: Agenda: 1. Decomposition Lab (10 minutes) 2. Finish Nitrogen Passport Activity Learning Goal: Students will understand make connections with the terms and concepts specific to ecology, biogeochemical cycle and the atmosphere.

Edible Aquifer Grocery List • At the station you are assigned, you as a group are responsible for bringing in the items as listed. • Assign who in your group will bring what items. • Items need to be brought in by Tuesday, February 19 th. • Make sure items are labeled with your name and period!!!!! • IF you do not bring your items you will have to go on a scavenger hunt and find others to borrow from or watch but do not get to eat! • Blue/red food coloring • 2 cans of Clear soda pop (7 Up, Sprite, etc) • Small gummy bears, (1 bag) • 2 cups of chocolate chips, • 10 crushed cookies, • Variety of colored cake decoration sprinkles and sugars ( 1 container) • Clear drinking straws (5) • Large clear plastic cups (5) • Spoons (5)

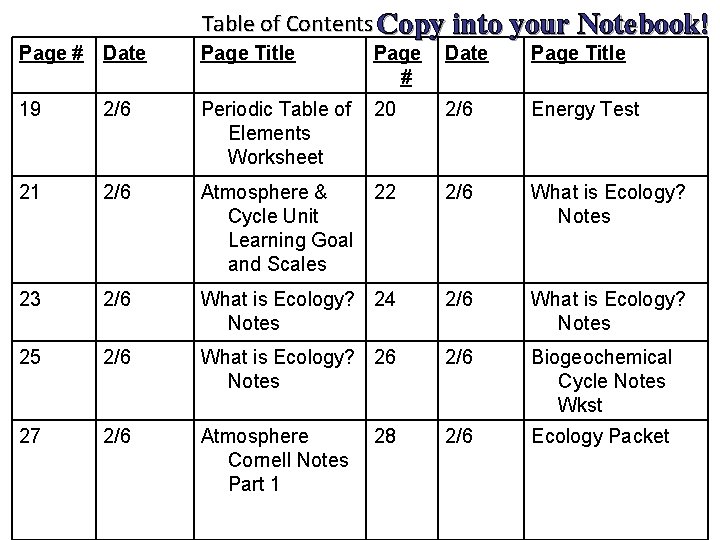
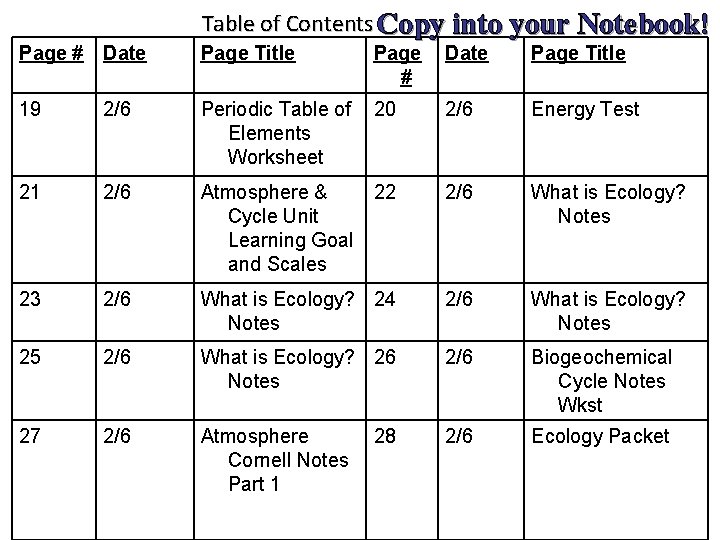
Table of Contents Copy into your Notebook! Page # Date Page Title 19 2/6 Periodic Table of Elements Worksheet 20 2/6 Energy Test 21 2/6 Atmosphere & Cycle Unit Learning Goal and Scales 22 2/6 What is Ecology? Notes 23 2/6 What is Ecology? 24 Notes 2/6 What is Ecology? Notes 25 2/6 What is Ecology? 26 Notes 2/6 Biogeochemical Cycle Notes Wkst 27 2/6 Atmosphere Cornell Notes Part 1 2/6 Ecology Packet 28

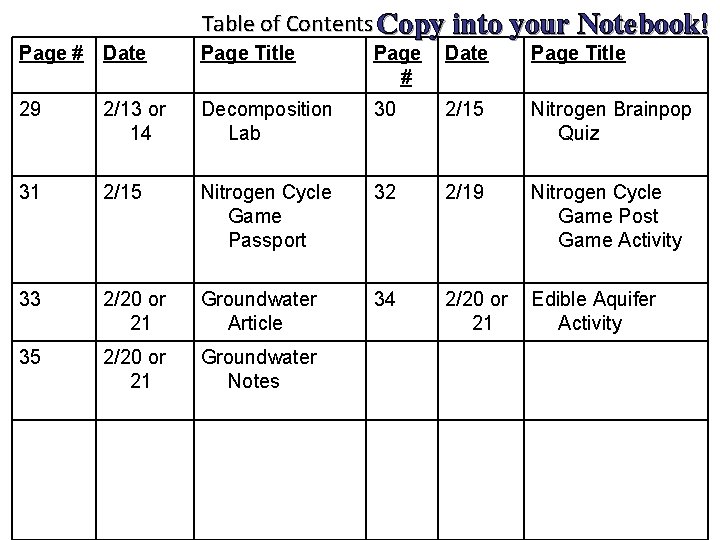
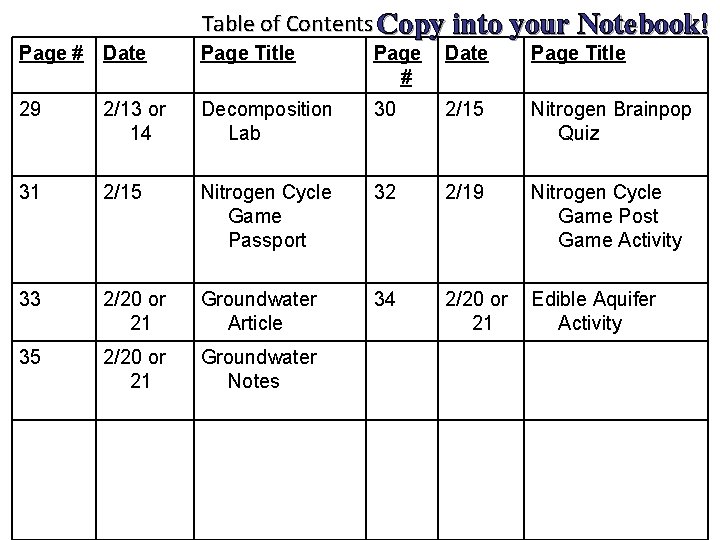
Table of Contents Copy into your Notebook! Page # Date Page Title 29 2/13 or 14 Decomposition Lab 30 2/15 Nitrogen Brainpop Quiz 31 2/15 Nitrogen Cycle Game Passport 32 2/19 Nitrogen Cycle Game Post Game Activity 33 2/20 or 21 Groundwater Article 34 2/20 or 21 Edible Aquifer Activity 35 2/20 or 21 Groundwater Notes

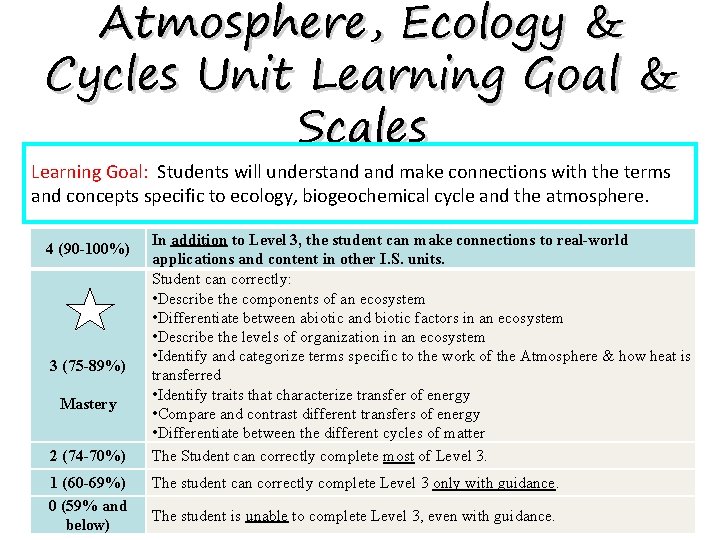
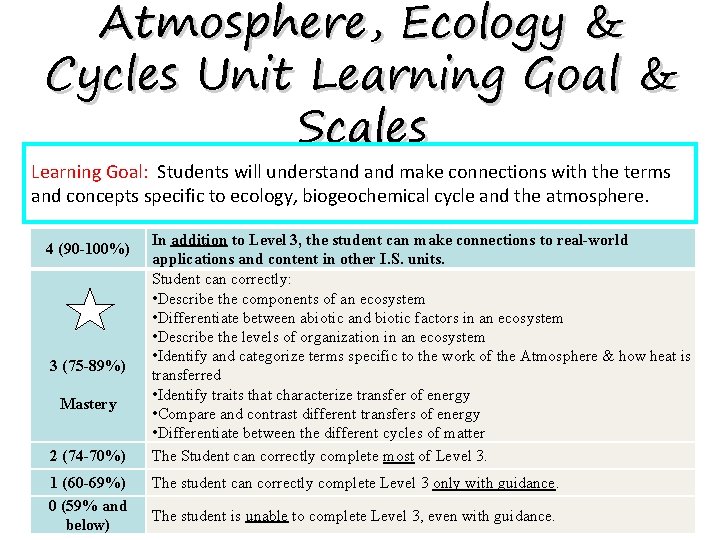
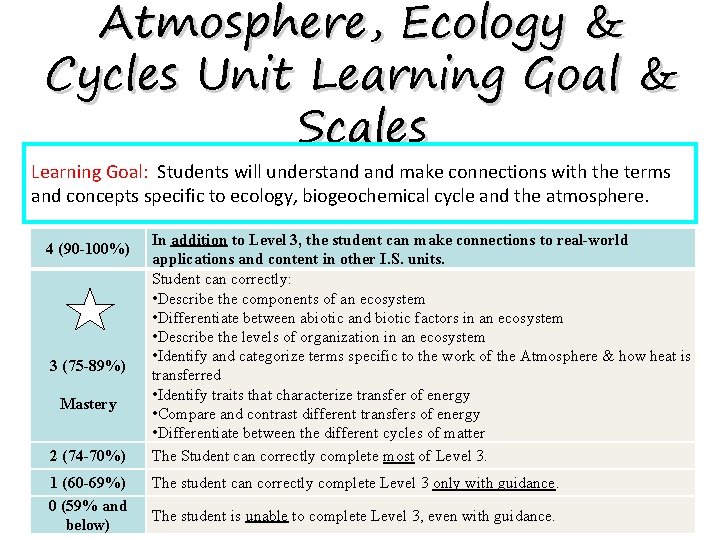
Atmosphere, Ecology & Cycles Unit Learning Goal & Scales Learning Goal: Students will understand make connections with the terms and concepts specific to ecology, biogeochemical cycle and the atmosphere. 4 (90 -100%) 3 (75 -89%) Mastery 2 (74 -70%) 1 (60 -69%) 0 (59% and below) In addition to Level 3, the student can make connections to real-world applications and content in other I. S. units. Student can correctly: • Describe the components of an ecosystem • Differentiate between abiotic and biotic factors in an ecosystem • Describe the levels of organization in an ecosystem • Identify and categorize terms specific to the work of the Atmosphere & how heat is transferred • Identify traits that characterize transfer of energy • Compare and contrast different transfers of energy • Differentiate between the different cycles of matter The Student can correctly complete most of Level 3. The student can correctly complete Level 3 only with guidance. The student is unable to complete Level 3, even with guidance.

Decompositio n Lab 1. 2. 3. 4. Open both jars. Record appearance of food scraps. Clean stations. Finish Lab outline.

The Nitrogen Cycle Post Game Activity V Symes

Today in I. S. … Week #1 (2/18 -2/22) Warm Up – Wednesday/Thursday (2/20 & 2/21): Pick up: • • Edible Aquifer Activity Worksheet Groundwater Worksheet Homework: Agenda: 1. Finish Carbon Cycle Notes 2. Groundwater Reading 3. Edible Aquifer Activity Learning Goal: Students will understand make connections with the terms and concepts specific to ecology, biogeochemical cycle and the atmosphere.

Edible Aquifer Grocery List • At the station you are assigned, you as a group are responsible for bringing in the items as listed. • Assign who in your group will bring what items. • Items need to be brought in by Tuesday, February 19 th. • Make sure items are labeled with your name and period!!!!! • IF you do not bring your items you will have to go on a scavenger hunt and find others to borrow from or watch but do not get to eat! • Blue/red food coloring • 2 cans of Clear soda pop (7 Up, Sprite, etc) • Small gummy bears, (1 bag) • 2 cups of chocolate chips, • 10 crushed cookies, • Variety of colored cake decoration sprinkles and sugars ( 1 container) • Clear drinking straws (5) • Large clear plastic cups (5) • Spoons (5)

Table of Contents Copy into your Notebook! Page # Date Page Title 19 2/6 Periodic Table of Elements Worksheet 20 2/6 Energy Test 21 2/6 Atmosphere & Cycle Unit Learning Goal and Scales 22 2/6 What is Ecology? Notes 23 2/6 What is Ecology? 24 Notes 2/6 What is Ecology? Notes 25 2/6 What is Ecology? 26 Notes 2/6 Biogeochemical Cycle Notes Wkst 27 2/6 Atmosphere Cornell Notes Part 1 2/6 Ecology Packet 28

Table of Contents Copy into your Notebook! Page # Date Page Title 29 2/13 or 14 Decomposition Lab 30 2/15 Nitrogen Brainpop Quiz 31 2/15 Nitrogen Cycle Game Passport 32 2/19 Nitrogen Cycle Game Post Game Activity 33 2/20 or 21 Groundwater Article 34 2/20 or 21 Edible Aquifer Activity 35 2/20 or 21 Groundwater Notes

Atmosphere, Ecology & Cycles Unit Learning Goal & Scales Learning Goal: Students will understand make connections with the terms and concepts specific to ecology, biogeochemical cycle and the atmosphere. 4 (90 -100%) 3 (75 -89%) Mastery 2 (74 -70%) 1 (60 -69%) 0 (59% and below) In addition to Level 3, the student can make connections to real-world applications and content in other I. S. units. Student can correctly: • Describe the components of an ecosystem • Differentiate between abiotic and biotic factors in an ecosystem • Describe the levels of organization in an ecosystem • Identify and categorize terms specific to the work of the Atmosphere & how heat is transferred • Identify traits that characterize transfer of energy • Compare and contrast different transfers of energy • Differentiate between the different cycles of matter The Student can correctly complete most of Level 3. The student can correctly complete Level 3 only with guidance. The student is unable to complete Level 3, even with guidance.

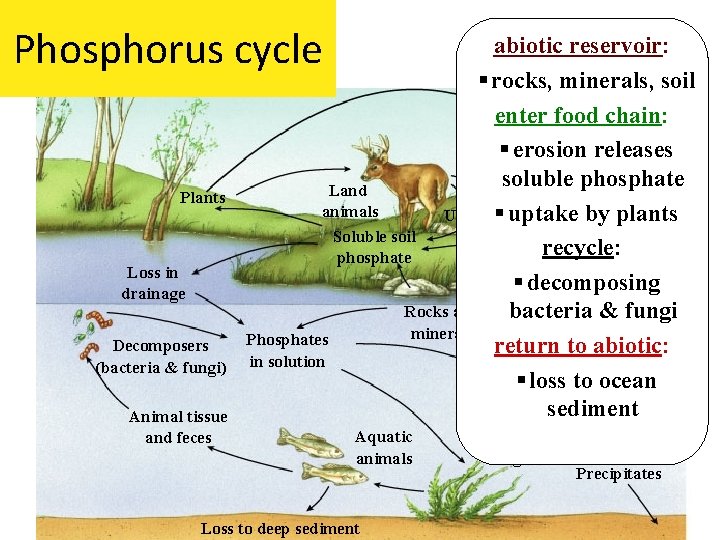
Phosphorus cycle Plants Loss in drainage Decomposers (bacteria & fungi) Animal tissue and feces abiotic reservoir: § rocks, minerals, soil enter food chain: § erosion releases soluble phosphate Land Animal tissue animals Urine § uptake by plants and feces Soluble soil Decomposers recycle: phosphate (bacteria and § decomposing fungi) Rocks and bacteria & fungi minerals Phosphates return to abiotic: in solution § loss to ocean sediment Aquatic animals Loss to deep sediment Plants and algae Precipitates

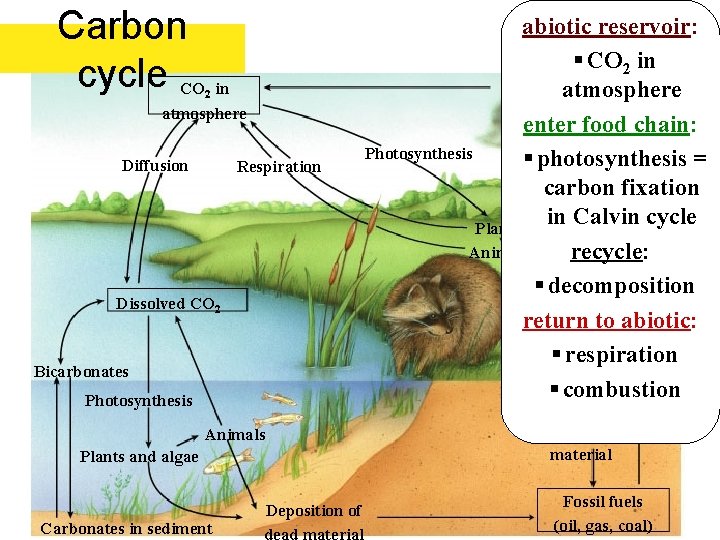
Carbon cycle CO in 2 atmosphere Diffusion Respiration Dissolved CO 2 Bicarbonates Photosynthesis Deposition of dead material Animals Plants and algae Deposition of Carbonates in sediment abiotic reservoir: § CO 2 in Combustion of fuels atmosphere enter food chain: Industry and home Photosynthesis § photosynthesis = carbon fixation in Calvin cycle Plants Animals recycle: § decomposition return to abiotic: § respiration § combustion Fossil fuels (oil, gas, coal)

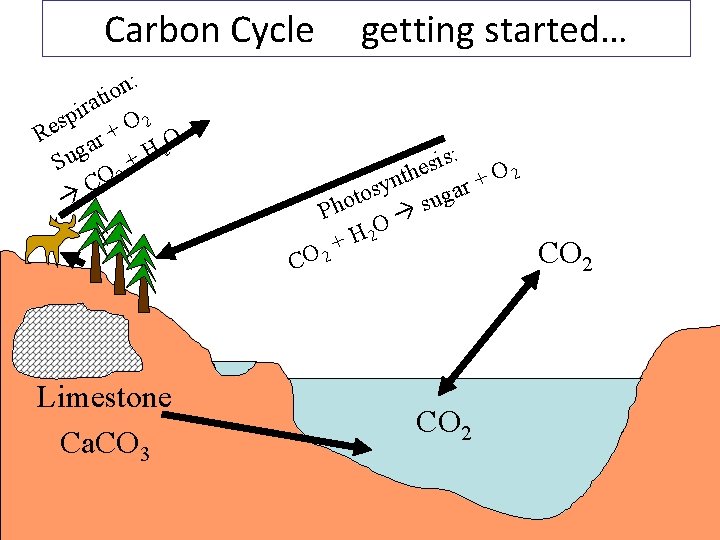
Carbon Cycle Most of the carbon on earth is locked up in the crust in limestone rocks: Ca. CO 3

Some of the rest is in fossil fuels: coal, crude oil and natural gas. Coal = carbon Methane = CH 4

Some is in the atmosphere as CO 2

A lot is dissolved in seawater as carbonate (CO 32 -), bicarbonate (HCO 3 -), carbonic acid (H 2 CO 3) and carbon dioxide (CO 2)

And of course some is in living & dead biological tissue (Biomass)

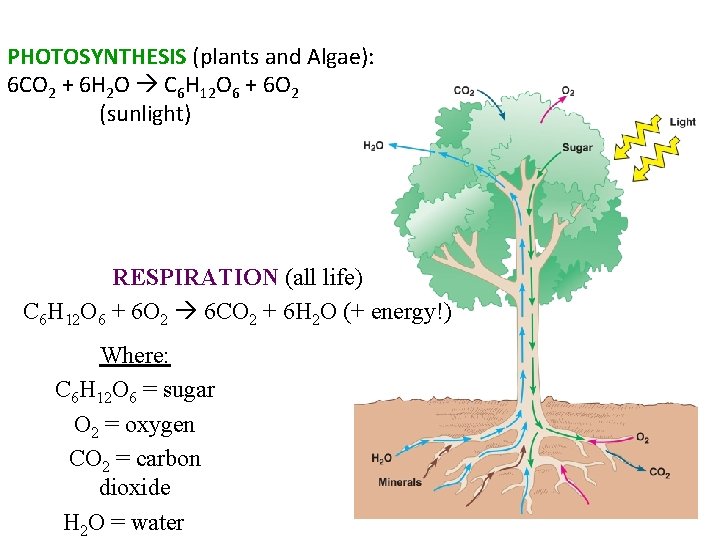
PHOTOSYNTHESIS (plants and Algae): 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 (sunlight) RESPIRATION (all life) C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O (+ energy!) Where: C 6 H 12 O 6 = sugar O 2 = oxygen CO 2 = carbon dioxide H 2 O = water

Carbon Cycle : n o ti a r i p 2 s O e + R O r a 2 g +H Su CO 2 getting started… : s i s 2 he O t n + r sy a o g t u s Pho O + H 2 CO 2 Limestone Ca. CO 3 CO 2

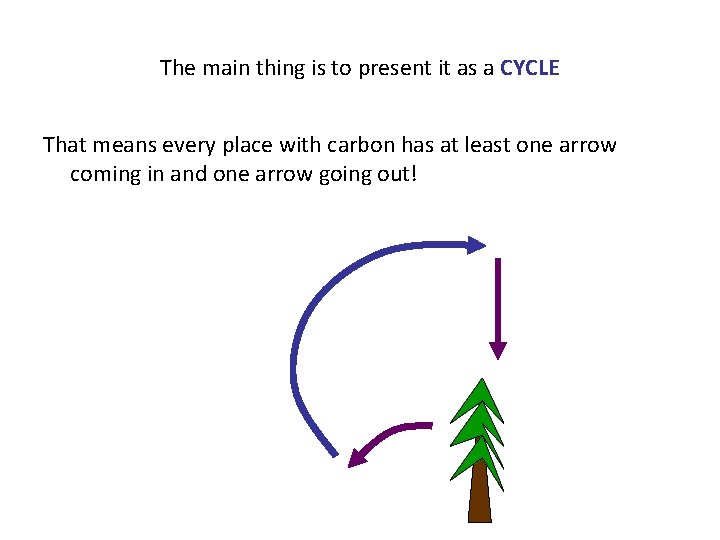
The main thing is to present it as a CYCLE That means every place with carbon has at least one arrow coming in and one arrow going out!

This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.

12 Minutes to read article! Show teacher for a stamp!

Edible Aquifer Lab… you never knew the Earth was so yummy! RULES for this LAB • Do NOT touch or eat ANYTHING on your lab station until told to by ME! • You MUST follow the DIRECTIONS step-by-step to do this CORRECTLY! – Do NOT shake or swirl your Parfait! • If at ANY TIME the privileges of the lab are ABUSED, I WILL STOP the lab! • Before you start, you will need to answer the pre-lab questions and get a stamp from me indicating you are ready to move on to the next step.

Edible Aquifer Lab • Do NOT shake or swirl your parfait! • Do NOT eat it until you are COMPLETELY FINISHED answering Questions 1 -10! (Use your “What in the Well is Groundwater? ” worksheet to help you with the questions. ) • To clean up: – Small plastic cups & dirty spoons need to be washed with soap and rinsed out – Your soda Recycle in the LARGE bin – Throw away everything else and wash down your lab station! – YOU WILL NOT BE DISMISSED TO THE FRONT OF THE ROOM UNTIL I HAVE CHECKED YOUR STATION! – ONE RULE BROKEN WILL BE A REFERRAL!



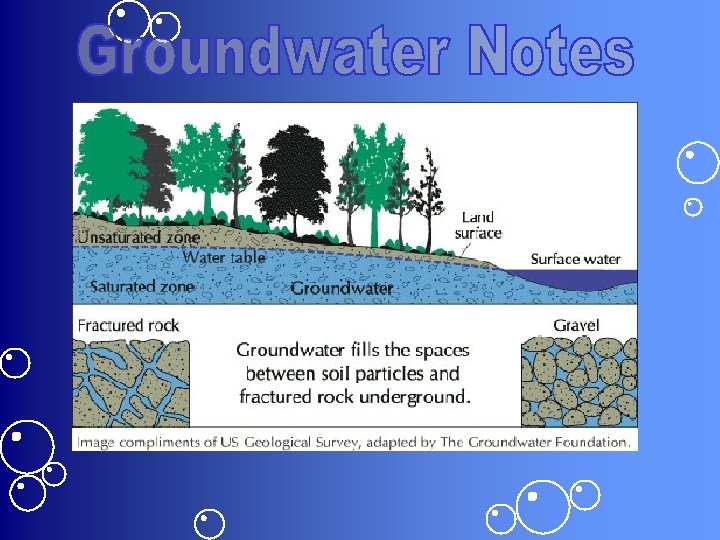
GROUNDWATER When rain falls to the ground, the water does not stop moving. Some of it flows along the surface to streams or lakes, some of it is used by plants, some evaporates and returns to the atmosphere, and some sinks into the ground. Imagine pouring a glass of water onto a pile of sand. Where does the water go? The water moves into the spaces between the particles of sand.


Groundwater is water that is found underground in the cracks and spaces in soil, sand rock. Groundwater is stored in--and moves slowly through--layers of soil, sand rocks called aquifers. Aquifers typically consist of gravel, sandstone, or fractured rock, like limestone. These materials are permeable because they have large connected spaces that allow water to flow through. The speed at which groundwater flows depends on the size of the spaces in the soil or rock and how well the spaces are connected.

The area where water fills the aquifer is called the saturated zone (or saturation zone). The top of this zone is called the water table. The water table may be located only a foot below the ground’s surface or it can sit hundreds of feet down. Groundwater can be found almost everywhere. The water table may be deep or shallow; and may rise or fall depending on many factors. Heavy rains or melting snow may cause the water table to rise, or heavy pumping of groundwater supplies may cause the water table to fall.

Water in aquifers is brought to the surface naturally through a spring or can be discharged into lakes and streams. Groundwater can also be extracted through a well drilled into the aquifer. A well is a pipe in the ground that fills with groundwater. This water can be brought to the surface by a pump. Shallow wells may go dry if the water table falls below the bottom of the well. Some wells, called artesian wells, do not need a pump because of

Groundwater supplies are replenished, or recharged, by rain and snow melt. In some areas of the world, people face serious water shortages because groundwater is used faster than it is naturally replenished. In other areas groundwater is polluted by human activities. In areas where material above the aquifer is permeable, pollutants can readily sink into groundwater supplies. Groundwater can be polluted by landfills, septic tanks, leaky underground gas tanks, and from overuse of fertilizers and pesticides. If groundwater becomes polluted, it will no longer be

Groundwater is used for drinking water by more than 50 percent of the people in the United States, including almost everyone who lives in rural areas. The largest use for groundwater is to irrigate crops. It is important for all of us to learn to protect our groundwater because of its importance as a source of water for drinking and irrigation. http: //www. groundwater. org/kc/kidsvocab. html

Today in I. S. … Week #1 (2/18 -2/22) Warm Up – Friday (2/22): Agenda: 1. Finish Decomposition Lab Pick up: Nothing Homework: Decomposition Lab due Friday, March 8 th for 30 points (Lab Sample) Learning Goal: Students will understand make connections with the terms and concepts specific to ecology, biogeochemical cycle and the atmosphere.

Edible Aquifer Grocery List • At the station you are assigned, you as a group are responsible for bringing in the items as listed. • Assign who in your group will bring what items. • Items need to be brought in by Tuesday, February 19 th. • Make sure items are labeled with your name and period!!!!! • IF you do not bring your items you will have to go on a scavenger hunt and find others to borrow from or watch but do not get to eat! • Blue/red food coloring • 2 cans of Clear soda pop (7 Up, Sprite, etc) • Small gummy bears, (1 bag) • 2 cups of chocolate chips, • 10 crushed cookies, • Variety of colored cake decoration sprinkles and sugars ( 1 container) • Clear drinking straws (5) • Large clear plastic cups (5) • Spoons (5)

Table of Contents Copy into your Notebook! Page # Date Page Title 19 2/6 Periodic Table of Elements Worksheet 20 2/6 Energy Test 21 2/6 Atmosphere & Cycle Unit Learning Goal and Scales 22 2/6 What is Ecology? Notes 23 2/6 What is Ecology? 24 Notes 2/6 What is Ecology? Notes 25 2/6 What is Ecology? 26 Notes 2/6 Biogeochemical Cycle Notes Wkst 27 2/6 Atmosphere Cornell Notes Part 1 2/6 Ecology Packet 28

Table of Contents Copy into your Notebook! Page # Date Page Title 29 2/13 or 14 Decomposition Lab 30 2/15 Nitrogen Brainpop Quiz 31 2/15 Nitrogen Cycle Game Passport 32 2/19 Nitrogen Cycle Game Post Game Activity 33 2/20 or 21 Groundwater Article 34 2/20 or 21 Edible Aquifer Activity 35 2/20 or 21 Groundwater Notes

Atmosphere, Ecology & Cycles Unit Learning Goal & Scales Learning Goal: Students will understand make connections with the terms and concepts specific to ecology, biogeochemical cycle and the atmosphere. 4 (90 -100%) 3 (75 -89%) Mastery 2 (74 -70%) 1 (60 -69%) 0 (59% and below) In addition to Level 3, the student can make connections to real-world applications and content in other I. S. units. Student can correctly: • Describe the components of an ecosystem • Differentiate between abiotic and biotic factors in an ecosystem • Describe the levels of organization in an ecosystem • Identify and categorize terms specific to the work of the Atmosphere & how heat is transferred • Identify traits that characterize transfer of energy • Compare and contrast different transfers of energy • Differentiate between the different cycles of matter The Student can correctly complete most of Level 3. The student can correctly complete Level 3 only with guidance. The student is unable to complete Level 3, even with guidance.

Decompositio n Lab 1. 2. 3. 4. Open both jars. Record appearance of food scraps. Clean stations. Finish Lab outline.

Intro to the Atmosphere Lesson Sections • Atmospheric Properties • Structure of the Atmosphere • Atmospheric Processes

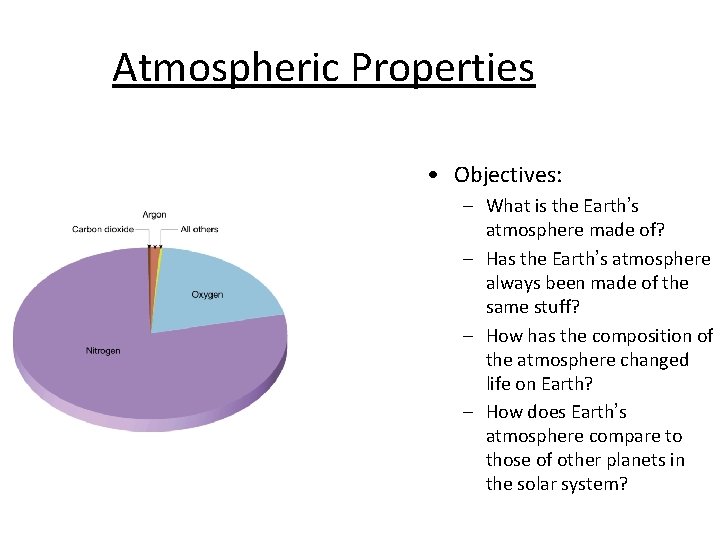
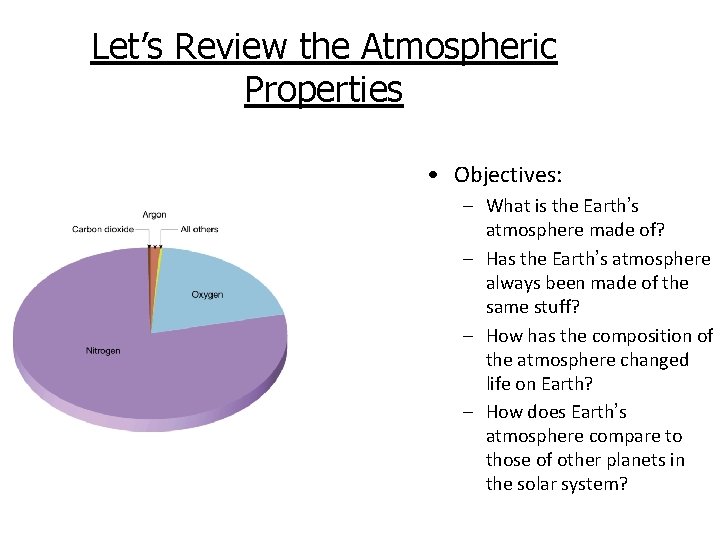
Atmospheric Properties • Objectives: – What is the Earth’s atmosphere made of? – Has the Earth’s atmosphere always been made of the same stuff? – How has the composition of the atmosphere changed life on Earth? – How does Earth’s atmosphere compare to those of other planets in the solar system?

What is the Atmosphere? • Atmosphere – Very thin envelope of gases that surrounds Earth – Used by living organisms for chemical compounds/nutrients (I. e. , O 2, CO 2, H 2 O, N 2) – Has no outer boundary, just fades into space

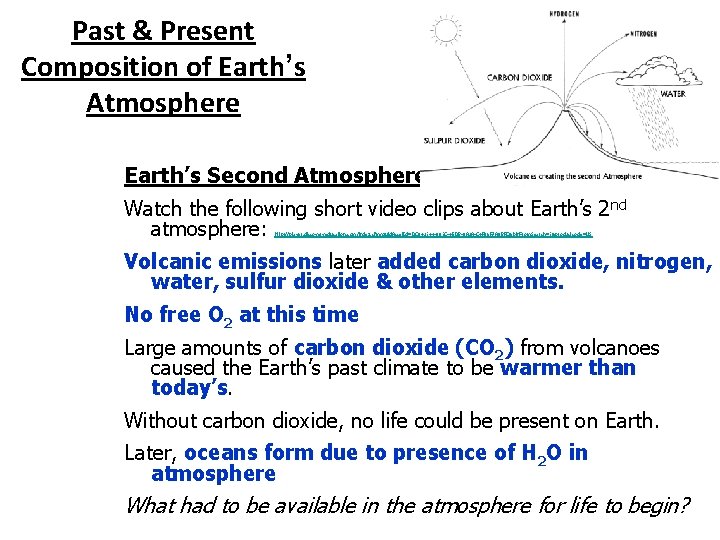
Past & Present Composition of Earth’s Atmosphere The composition of the Earth’s atmosphere has changed since the formation of the Earth. How old is the Earth according to the Nebular Hypothesis? Earth has had approximately 3 different atmospheres over the course of over 4 billion years. What gases made up Earth’s original atmosphere? Earth’s First Atmosphere The Earth’s first atmosphere was mainly helium (He) & hydrogen (H). Why?

Past & Present Composition of Earth’s Atmosphere Earth’s Second Atmosphere Watch the following short video clips about Earth’s 2 nd atmosphere: Volcanic emissions later added carbon dioxide, nitrogen, water, sulfur dioxide & other elements. No free O 2 at this time Large amounts of carbon dioxide (CO 2) from volcanoes caused the Earth’s past climate to be warmer than today’s. Without carbon dioxide, no life could be present on Earth. Later, oceans form due to presence of H 2 O in atmosphere What had to be available in the atmosphere for life to begin? http: //player. discoveryeducation. com/index. cfm? guid. Asset. Id=DC 842144 -801 C-45 DB-8 A 2 A-C 4 F 06 F 7 A 8 BFC&bln. From. Search=1&productcode=US

Past & Present Composition of Earth’s Atmosphere Today’s Atmosphere – – – Nitrogen (N 2)- 78% Oxygen (O 2)- 21% Argon – 0. 9% Carbon Dioxide (CO 2) - 0. 03% Other miscellaneous gases (I. e. , H 2 O) - 0. 07% – Using this information, devise a graph/chart to show this quantitative data. • Don’t forget to label & color each piece of data!

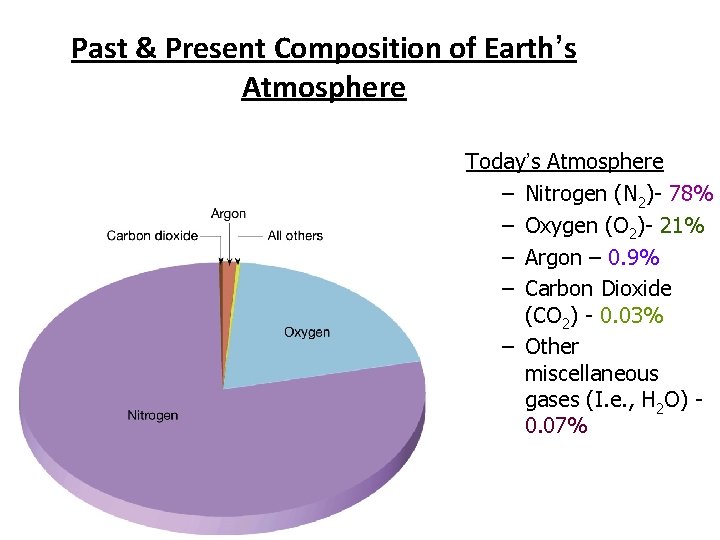
Past & Present Composition of Earth’s Atmosphere Today’s Atmosphere – Nitrogen (N 2)- 78% – Oxygen (O 2)- 21% – Argon – 0. 9% – Carbon Dioxide (CO 2) - 0. 03% – Other miscellaneous gases (I. e. , H 2 O) 0. 07%

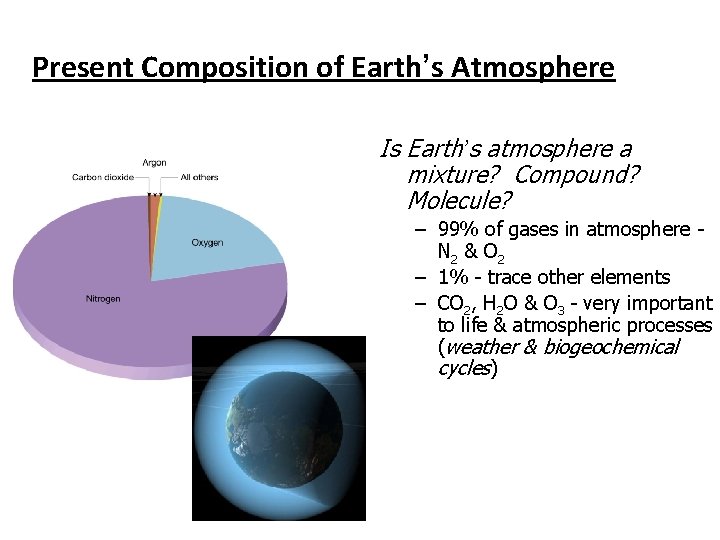
Present Composition of Earth’s Atmosphere Is Earth’s atmosphere a mixture? Compound? Molecule? – 99% of gases in atmosphere N 2 & O 2 – 1% - trace other elements – CO 2, H 2 O & O 3 - very important to life & atmospheric processes (weather & biogeochemical cycles)


Earth’s Atmosphere Vs. Other Planets’ Atmospheres Why has life (in intelligent, multi-cellular forms) NOT been found on other planets in our solar system? – The answer to this question might lie in comparing Earth’s atmosphere to other planets’ atmospheres…

The Goldilocks Principle: A Comparison of Planet’s Atmospheres lab • Read through the Background Information • Write your answers to the following questions under “Summarize this Background Information” – Why is understanding the composition of Earth’s atmosphere important? – Is the “greenhouse effect” good or bad for life on Earth? – What output of “life” keeps Earth’s atmosphere composition in balance?

The Goldilocks Principle: A Comparison of Planet’s Atmospheres lab • What are the Lab Objectives? • What is the Goal of each group? – How are you going to turn a percentage into a whole number (of marshmallows)? • 100% (composition) = 10 marshmallow – What percentage of Venus’ atmosphere is from CO 2? • 96. 5% = ? ? ? • 9. 65 pink marshmallows – FYI, you have 30 of each color marshmallow (for all 3 models). • Do not lose, drop OR eat ANY marshmallows! formed

The Goldilocks Principle: A Comparison of Planet’s Atmospheres lab • Data Observations – Color-code the Data Table (do you which color represents each gas) – Write in each Planet’s name under column in the designated color – Line up your marshmallows according to data table & draw the arrangement “Drawing of marshmallows row/bag” – Finally, turn your model into a Pie under “Graph” • Label & color-code each section! know “Planet” the under in a Chart

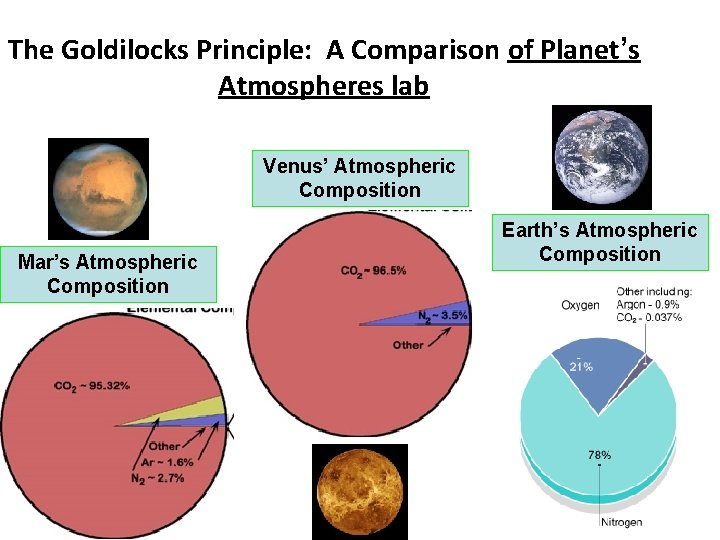
The Goldilocks Principle: A Comparison of Planet’s Atmospheres lab Venus’ Atmospheric Composition Mar’s Atmospheric Composition Earth’s Atmospheric Composition

Let’s Review the Atmospheric Properties • Objectives: – What is the Earth’s atmosphere made of? – Has the Earth’s atmosphere always been made of the same stuff? – How has the composition of the atmosphere changed life on Earth? – How does Earth’s atmosphere compare to those of other planets in the solar system?

Thurs/Fri. , March 1 & 2, 2012 • Lesson – – Layers of the atmosphere Review Worksheet - (6 th hour ONLY) • Read, answer the questions, discuss as class (25 minutes) – Bill Nye: The Water Video and Worksheet - (6 th hour ONLY) • (23 minutes long) • Discuss and collect worksheet when done (7 minutes) – Bill Nye: The Water Video Quiz – Intro to the Earth’s Atmosphere Notes – Goldilocks Lab

• Lesson – – Layers of the atmosphere Review Worksheet • Read, answer the questions, discuss as class (25 minutes) – Bill Nye: The Water Video and Worksheet • (23 minutes long) • Discuss and collect worksheet when done (7 minutes)

Sample Story Rosie the Water Molecule I knew I could do it! Just if I moved around a little more, flapped my molecule wings a little harder, I knew that eventually, I would evaporate up, up and away from here! And I did! Higher and higher I evaporated, until I was up in the atmosphere! From way up there, all my friends down in the ocean looked like little molecules. . . which, actually, they were. Once I was finally up in the atmosphere, it was like the greatest roller coaster ever. I was transported to all sorts of amazing places with a whole tour group of other water molecules. We called ourselves VAPOR, because the group consisted of Velma, Adam, Paul, Oren, and me, Rosie. We all became really close in our tour. So close, in fact, that after we were done traveling around the atmosphere, we fused together, and in a bond of friendship known to some as condensation, we became liquidized. It was so much fun! After such high paced movement, it was great to relax to a lower energy state. We knew that the atmosphere had been fun, but it had taken a lot out of us, so we fell back to earth in our condensed form. On the trip home, we saw all the giants pull out crazy contraptions that they called “umbrellas. ” We were careful to avoid those! When we reached earth’s surface, we knew that we had to go our separate ways. Some of us decided to runoff into unknown land. Others decided to take a tour of underground, on something called the Infiltration/ Percolation Train. I think I’ll join them. I had so much fun on this trip, who knows, I may do it again sometime.