What is the stimulus for erythropoiesis The most
























- Slides: 34

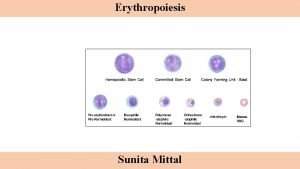
What is the stimulus for erythropoiesis? • The most potent stimulus for erythropoiesis is hypoxia. In hypoxia oxygen supply to tissues is decreased. • Erythropoiesis is stimulated by the production of a substance called “erythropoietin” • Erythropoietin is a glycoprotein, secreted mainly by kidneys(85%) and also to certain extent by liver(15%). Its half life is about 5 hrs

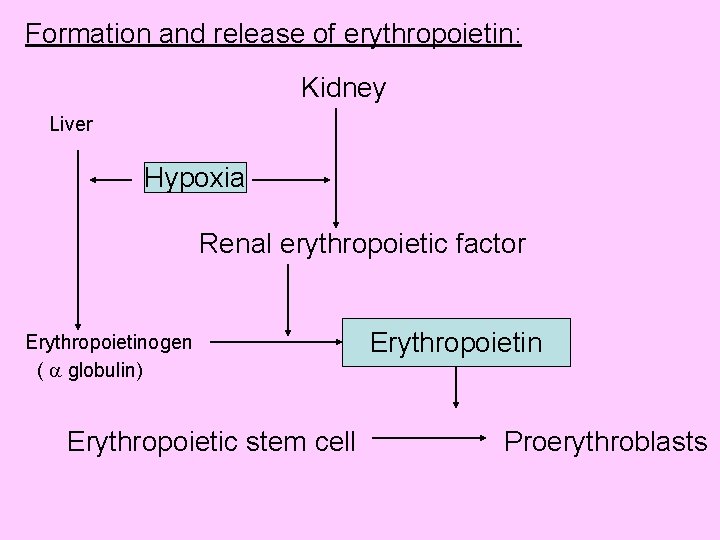
Formation and release of erythropoietin: Kidney Liver Hypoxia Renal erythropoietic factor Erythropoietinogen ( globulin) Erythropoietin Erythropoietic stem cell Proerythroblasts

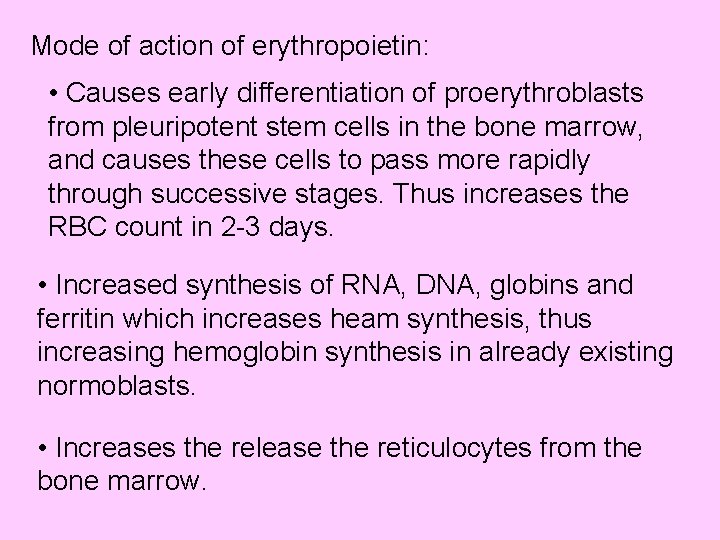
Mode of action of erythropoietin: • Causes early differentiation of proerythroblasts from pleuripotent stem cells in the bone marrow, and causes these cells to pass more rapidly through successive stages. Thus increases the RBC count in 2 -3 days. • Increased synthesis of RNA, DNA, globins and ferritin which increases heam synthesis, thus increasing hemoglobin synthesis in already existing normoblasts. • Increases the release the reticulocytes from the bone marrow.

Factors necessary for erythropoiesis: 1. General factors— Erythropoietin Hormones-testosterone, thyroxin, growth hormone and estrogen Hemopoietic growth factor Colony stimulating factor Vitamins 2. Maturation factors— Vitamin B 12 , intrinsic factor and folic acid 3. Factors for Hb synthesis— Proteins and amino acids, iron, copper, cobalt and nickel , vitamins.

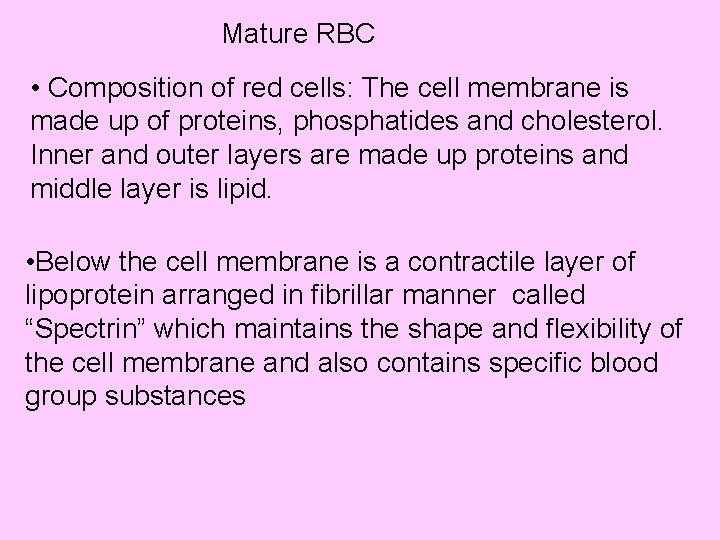
Mature RBC • Composition of red cells: The cell membrane is made up of proteins, phosphatides and cholesterol. Inner and outer layers are made up proteins and middle layer is lipid. • Below the cell membrane is a contractile layer of lipoprotein arranged in fibrillar manner called “Spectrin” which maintains the shape and flexibility of the cell membrane and also contains specific blood group substances

• The cell has stroma made up of lipoprotein containing hemoglobin. The inner material is in the form of a jelly. • The mature RBC has no nucleus, no mitochondria and no ribosomes. • Its cytoplasm contains all the enzymes necessary for metabolism of glucose and other substances, for utilization of oxygen. • These metabolic systems become progressively less active, which limits the life span of RBC • Normal life span of RBC is 120 days.


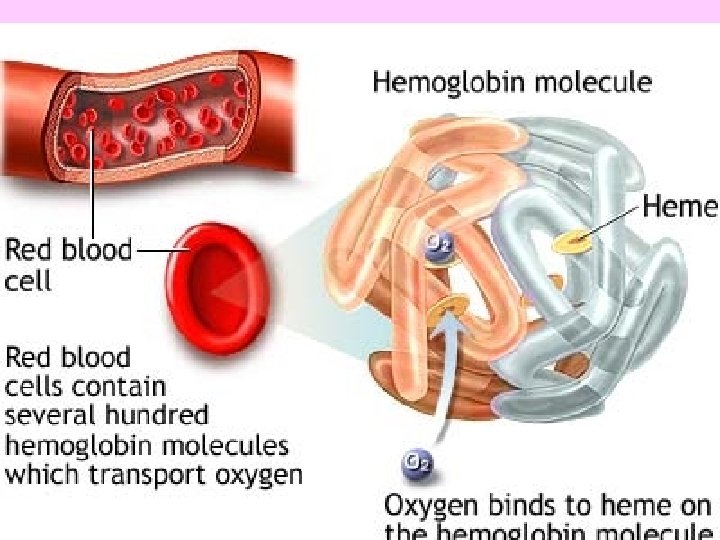
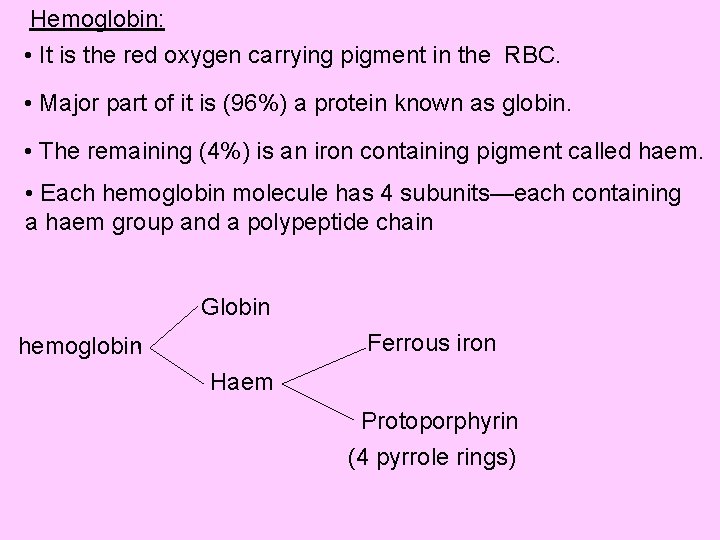
Hemoglobin: • It is the red oxygen carrying pigment in the RBC. • Major part of it is (96%) a protein known as globin. • The remaining (4%) is an iron containing pigment called haem. • Each hemoglobin molecule has 4 subunits—each containing a haem group and a polypeptide chain Globin Ferrous iron hemoglobin Haem Protoporphyrin (4 pyrrole rings)

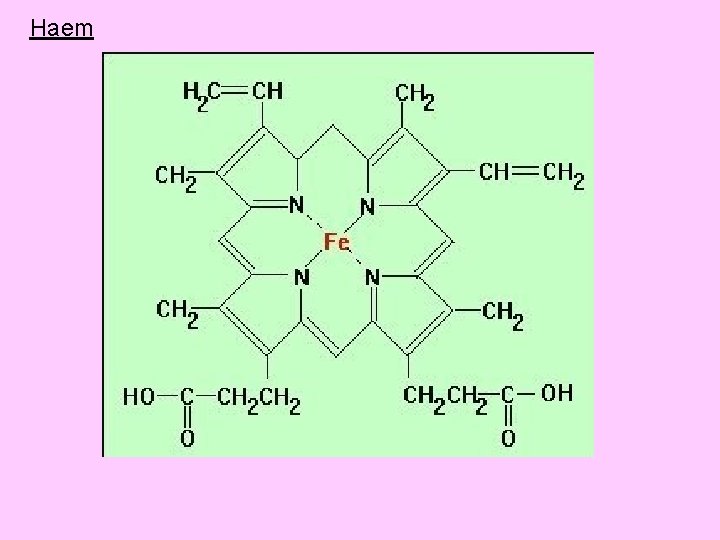
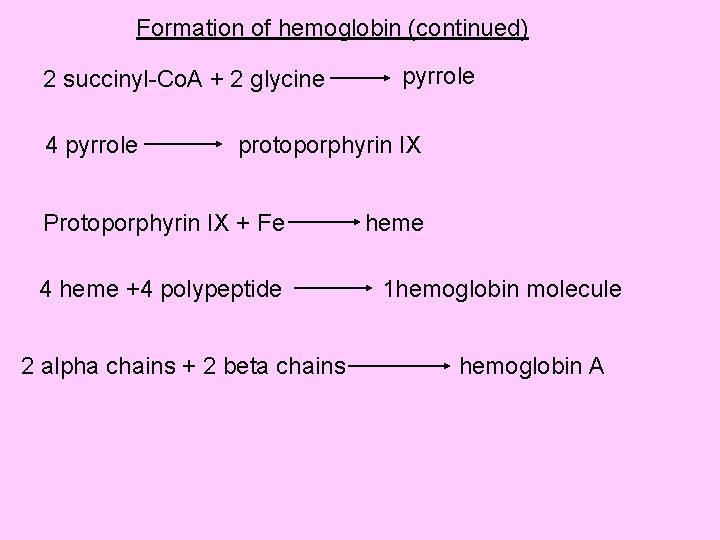
Haem is an iron containing porphyrin called iron-protoporphyrin IX • The protoporphyrin nucleus is a tetrapyrrole (4 pyrrole rings) • Pyrrole ring is synthesized from acetic acid and glycine and most of the synthesis takes place in mitochondria. • Acetic acid is changed in Krebs cycle into succinyl-Co. A • 2 molecules of glycine with 2 molecules of succinyl-Co. A forms a pyrrole ring • The protoporphyrin combines with iron to form heme

Haem

Globin: • Is a protein made up of 4 polypeptide chains, each having molecular weight of 16000. Synthesized in ribosome. • Of four chains, two are ‘alpha’ chains each containing 141 amino acids; and two are ‘beta’ chains each containing 146 amino acids; in adult hemoglobin (Hb. A) • Each polypeptide chain is associated with the iron of haem group. • Thus, in one molecule of hemoglobin there are four haem groups and 4 polypeptide chains. So there are 4 iron atoms which can carry 4 molecules of oxygen (8 atoms).

Formation of hemoglobin (continued) 2 succinyl-Co. A + 2 glycine 4 pyrrole protoporphyrin IX Protoporphyrin IX + Fe 4 heme +4 polypeptide 2 alpha chains + 2 beta chains heme 1 hemoglobin molecule hemoglobin A

• Hemoglobin has molecular weight of 68, 000. • Normal Hb content in blood in adults is: In males: 14 to 18 gm% In females: 12 to 15 gm%. Functions of Hb: • Transport of oxygen from lungs to tissue • Transport of carbon dioxide from tissue to lungs • Being a protein it acts as an excellent acid base buffer and is responsible for 70%buffering power of whole blood.

Synthesis of hemoglobin: • It takes place only in the developing RBCs; starts in the proerythroblastic stage and continues till reticulocyte stage. • Heme portion is synthesized in mitochondria mainly and the globin chain in the ribosome. Factors necessary for Hb synthesis: • Dietary protein • Minerals: Iron – in formation of heme Copper – in promoting absorption, mobilization and utilization of iron cobalt – necessary for manufacture of vit. B 12 by bacterial action in the GIT lumen calcium – increases iron absorption from GIT

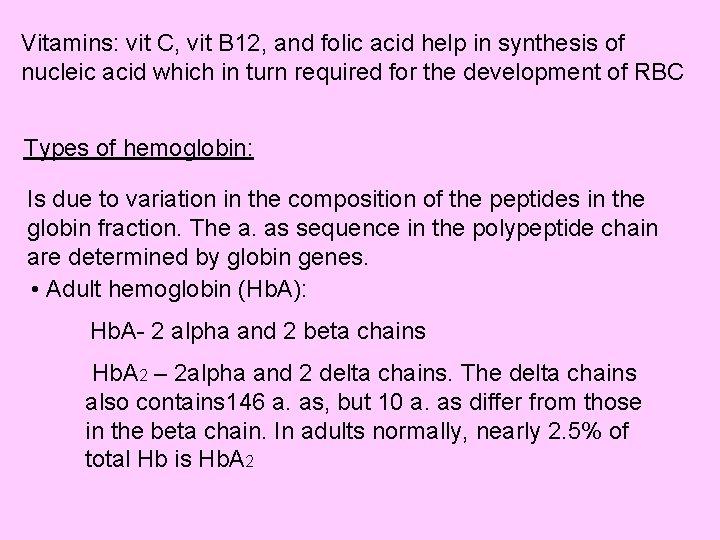
Vitamins: vit C, vit B 12, and folic acid help in synthesis of nucleic acid which in turn required for the development of RBC Types of hemoglobin: Is due to variation in the composition of the peptides in the globin fraction. The a. as sequence in the polypeptide chain are determined by globin genes. • Adult hemoglobin (Hb. A): Hb. A- 2 alpha and 2 beta chains Hb. A 2 – 2 alpha and 2 delta chains. The delta chains also contains 146 a. as, but 10 a. as differ from those in the beta chain. In adults normally, nearly 2. 5% of total Hb is Hb. A 2

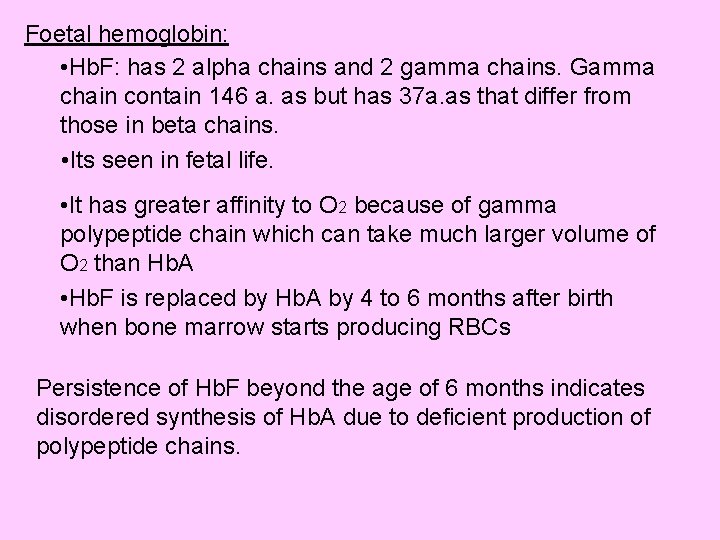
Foetal hemoglobin: • Hb. F: has 2 alpha chains and 2 gamma chains. Gamma chain contain 146 a. as but has 37 a. as that differ from those in beta chains. • Its seen in fetal life. • It has greater affinity to O 2 because of gamma polypeptide chain which can take much larger volume of O 2 than Hb. A • Hb. F is replaced by Hb. A by 4 to 6 months after birth when bone marrow starts producing RBCs Persistence of Hb. F beyond the age of 6 months indicates disordered synthesis of Hb. A due to deficient production of polypeptide chains.

Disorders due to abnormal Hb: • Thalassaemia: Is due to deficient production of alpha or beta chain. Thalassaemia alpha is very rare Thalassaemia beta is more common; and further divided into 2 types- major and minor Thalassaemia major is less common. It is homozygous in transmission and leads to moderate to severe anemia. There is total absence of beta chain synthesis and Hb. F levels are markedly increased Thalassaemia minor is more common. Heterozygous in transmission, leads to mild anemia. There is partial synthesis of beta chain.

Sickle cell anemia: • Here in each polypeptide chain of Hb. A, at position 6, a. as glutamic acid is replaced by valine, causing Hb. S • Hb. S is less soluble than Hb. A at low oxygen tension and Hb precipitates into elongated crystals which damages the cell membrane causing increased fragility of RBC. • Increased fragility of RBC leads to severe anemia. Other abnormal Hbs are Hb. C, E, I, J and M. All causes hemolytic anemia.

Derivatives of hemoglobin: Hb readily combines with gases or any other substances to form some products, which are called derivatives of Hb. • Oxyhemoglobin: In the presence of oxygen, Hb combines with oxygen by physical process of oxygenation. It is an unstable compound and the combination is reversible. • Reduced (deoxygenated) hemoglobin: This is when oxygen is released from the oxyhemoglobin. • Carbamino-Hemoglobin: is due to combination of carbon dioxide with globin

• Carboxy hemoglobin or carbon monoxy hemoglobin: CO reacts with Hb to produce this compound. The affinity of Hb for CO is 250 times more than the affinity for oxygen. Methemoglobin: When reduced or deoxygenated Hb is exposed to various drugs or oxidizing agents, the ferrous (Fe 2+) is oxidized to ferric(Fe 3+) form and the compound is called methemoglobin (Hb. OH). It cannot reversibly unite with gaseous oxygen

Destruction of Hb: After the life span of 120 days, the RBCs are destroyed in tissue macrophage system (reticulo- endothelial system) and Hb is degraded in these cells and split into globin, iron and porphyrin. • Globin enters the amino acid pool • Iron also enters the liver to be stored and reused for synthesis of hemoglobin • The porphyrin ring is oxidised to biliverdin and reduced to bilirubin due to the action of biliverdin reductase

Bilirubin is the yellow colored pigment, insoluble in water. Its called (free)/ unconjugated bilirubin; carried to liver being bound to albumin. In the hepatic cells it undergoes conjugation to form water soluble bilirubin Major part of the conjugated bilirubin passes through the bile duct into the intestine and as it reaches the large intestine it is degraded by the colonic bacteria to form colorless compound called (stercobilinogen), which is later oxidized to brownish compound which gives color to the faeces.

About 20% of stercobilinogen is reabsorbed into the circulation and reaches liver to be re-excreted in the bile— ‘enterohepatic circulation’ A small part of conjugated bilirubin escapes into general circulation and is excreted in urine.


Normal bilirubin concentration in plasma is less than 1 mg% Bilirubin level more than 2 mg% indicates ‘Jaundice’ Types of jaundice: 1. Pre hepatic (hemolytic) 2. Hepatic (hepatocellular) 3. Post hepatic (obstructive)

Tissue macrophages: (reticuloendothelial system) are the special group of phagocytic cells found in different parts of the body. They can phagocytose large foreign particles • Littoral cells – found in the lining of the blood sinuses in bone marrow • Kupffer cells – found along the vascular capillaries in the liver • Reticulum cells – found in red and white pulp of spleen • Pulmonary alveolar macrophages – in lungs • Osteoclasts in bones • Microglia in brain • Subcutaneous tissue

Functions of tissue macrophages: • They ingest and destroy the blood cells; form and release bilirubin. • Defence of the body against infection. • They ingest and process antigens which then stimulate antibody formation

Iron metabolism: Iron is necessary for the formation of – hemoglobin myoglobin peroxidase catalase cytochrome and cytochrome oxidase Myoglobin, peroxidase and catalase contain different protein groups bound to the same porphyrin as in heme –Iron protoporphyrin IX

Iron distribution in the body: The total quantity of iron in the body averages 4 to 5 gms. Approximate distribution is— 65% in hemoglobin 4% in myoglobin 1% in other heme compounds. 25 to 30% stored mainly in liver as ferritin and hemosiderrin. And 0. 1% combined with protein tansferrin in the plasma. Daily loss of iron: • in males amount of iron lost from the body is about 1 mg/day and in females its about 2 mg/day due to extra iron lost in blood shed during menstruation.

Iron requirement: For adult men its about— 5 to 10 mg/day For adult females about– 20 mg/day. Iron absorption: • The amount of iron absorbed is only 3 to 6% of the amount ingested. • Iron in foods of animal origin is better absorbed because it is in heme form and iron is in ferrous state. E g: Fish, meat chicken. • Nonheme iron available in vegetables, grains and cereals is in ferric form and must be reduced by Hcl secreted by the stomach or ascorbic acid into ferrous for better absorption. • Iron absorption is mainly in duodenum and upper jejunum.

• Iron absorption is an active process. • Phytic acid (found in cereals), Phosphates, oxalates, pancreatic juice react with iron to form insoluble compounds in the intestine and decrease iron absorption. • In the intestinal lumen, iron is bound to a protein called apotransferrin (formed in the liver) and transported across the brush border into the intestinal mucosal cells. • From the mucosal cells part of the iron is directly passed into blood stream and much of it is bound to a protein apoferritin to form “ferritin”– which is the principle storage form of iron in the intestine and other tissues.

• The iron stored in the intestinal mucosal cells is lost with the cells when they are shed into the lumen and passed in faeces. • Within the cells the ferritin molecules aggregate in the lysosomal membrane and these deposits are called “Hemosiderin”– an iron store Regulation of total body iron: Control of iron absorption by “intestinal mucosal theory”: Iron absorption into blood stream is increased when body iron stores are depleted, and when erythropoiesis is increased. In iron deficiency, the amount of circulating transferrin is increased so more iron moves from the cells to the transferrin, and less is stored.

Regulation of total body iron: Control of iron absorption is by intestinal mucosal cells. • Iron absorption into blood stream is increased when body iron stores are depleted, and • when erythropoiesis is increased. In iron deficiency, • the amount of circulating transferrin is increased and • its percent saturation with iron is decreased, so more iron moves from the mucosal cells to transferrin and less is stored.

In iron overload, the opposite of above occurs – • stoppage of apotransferrin formation from the liver, so that, iron could not be absorbed from the intestine; and • reduction of release of iron from the transferrin, so that, transferrin is completely saturated with iron and further absorption is prevented. The ability of the mucosa to prevents excess ingested iron being absorbed– “mucosal block” theory
 What is the stimulus for erythropoiesis
What is the stimulus for erythropoiesis Unit 6 learning ap psychology
Unit 6 learning ap psychology Learning is a relatively permanent change in
Learning is a relatively permanent change in Neutral stimulus becomes a conditioned stimulus
Neutral stimulus becomes a conditioned stimulus Factors necessary for erythropoiesis
Factors necessary for erythropoiesis Heme globin
Heme globin Factors affecting erythropoiesis
Factors affecting erythropoiesis Hgb bank
Hgb bank Erythropoiesis
Erythropoiesis Stages of erythropoisis
Stages of erythropoisis Brenda beckett
Brenda beckett Dr sri lestari
Dr sri lestari Erythropoiesis
ErythropoiesisErythropoiesis guyton
 Mesoblastic stage of erythropoiesis
Mesoblastic stage of erythropoiesis Erythropoiesis process
Erythropoiesis process Erythropoiesis
Erythropoiesis Erythroid precursors
Erythroid precursors Borra hål för knoppar
Borra hål för knoppar Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Bris för vuxna
Bris för vuxna Trög för kemist
Trög för kemist Teckenspråk minoritetsspråk argument
Teckenspråk minoritetsspråk argument Datorkunskap för nybörjare
Datorkunskap för nybörjare Autokratiskt ledarskap
Autokratiskt ledarskap Indikation för kejsarsnitt på moderns önskan
Indikation för kejsarsnitt på moderns önskan Toppslätskivling effekt
Toppslätskivling effekt Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Rita perspektiv
Rita perspektiv Redogör för vad psykologi är
Redogör för vad psykologi är Bästa kameran för astrofoto
Bästa kameran för astrofoto Mat för idrottare
Mat för idrottare Lek med geometriska former
Lek med geometriska former Offentlig förvaltning
Offentlig förvaltning Form dikt
Form dikt