Water and Solutions The importance of water Important


















- Slides: 23

Water and Solutions

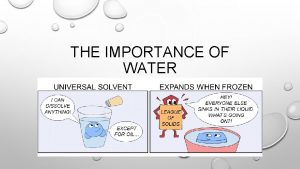
The importance of water • Important component of solutions • Water quality, composition, and p. H can drastically affect an experiment

Which water should I use? • Tap water ▫ Variable chemistry and purity • Laboratory grade water ▫ Reverse osmosis or distillation ▫ Rinsing glassware, media preparation • Reagent grade water ▫ Filtration, deionization, and carbon adsorption ▫ Most laboratory uses • Other specialized types (dd, microfiltered, electrically deionized, etc. )

Solution terminology • Solution: A mixture in which individual molecules or ions are dispersed in a liquid. • Solvent: The liquid that makes up the majority of the solution. ▫ e. g. , water (aqueous solution) • Solute: The minority component of the solution. Often a solid before mixing.

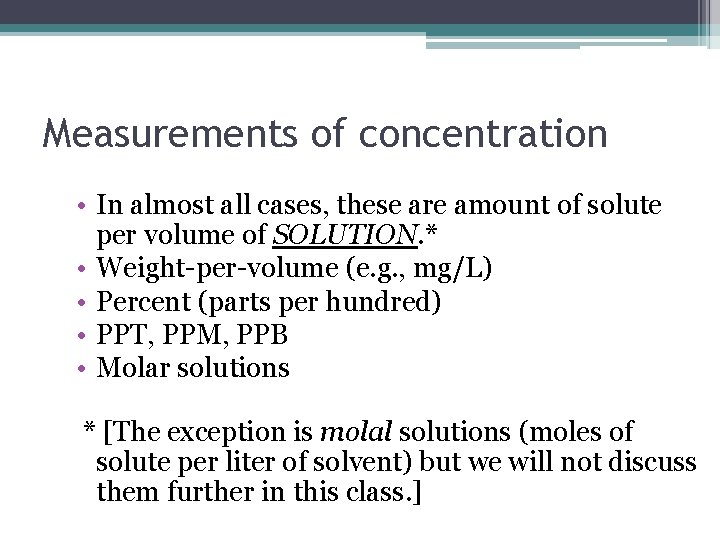
Measurements of concentration • In almost all cases, these are amount of solute per volume of SOLUTION. * • Weight-per-volume (e. g. , mg/L) • Percent (parts per hundred) • PPT, PPM, PPB • Molar solutions * [The exception is molal solutions (moles of solute per liter of solvent) but we will not discuss them further in this class. ]

Weight-per-volume concentration • g/L, µg/m. L, etc. • How to make up a 10 g/L aqueous solution: ▫ Weigh out 10 g of the substance. ▫ Put it in a vol. flask or grad. cylinder ▫ Add water up to the 1 L mark. • If you mix 1 L water with 10 g solute, then the total volume would be >1 L, and the concentration would be <10 g/L.

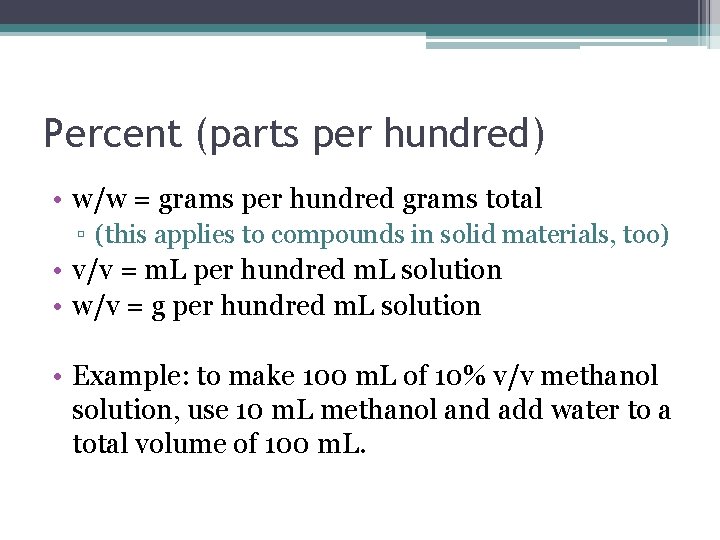
Percent (parts per hundred) • w/w = grams per hundred grams total ▫ (this applies to compounds in solid materials, too) • v/v = m. L per hundred m. L solution • w/v = g per hundred m. L solution • Example: to make 100 m. L of 10% v/v methanol solution, use 10 m. L methanol and add water to a total volume of 100 m. L.

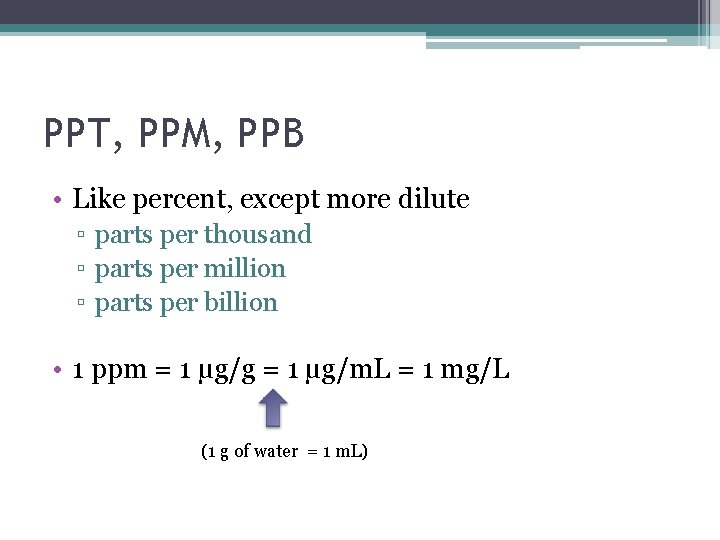
PPT, PPM, PPB • Like percent, except more dilute ▫ parts per thousand ▫ parts per million ▫ parts per billion • 1 ppm = 1 µg/g = 1 µg/m. L = 1 mg/L (1 g of water = 1 m. L)

Molar (M) Solutions • Definition: moles of solute per liter of solution ▫ 1 mole is 6 x 1023 molecules. ▫ The mass of 1 mole of a compound is the molecular weight (MW) of that compound • What you need to know: ▫ Volume desired ▫ Concentration desired ▫ Formula weight (FW) (FW = MW + water of hydration, if any)

• A 1 M solution is the FW of a substance in 1 liter of solution. • Example 1: ▫ Make up 1 liter of 1 M Na. Cl…


• Example 2: ▫ Make up 400 ml of 0. 25 M Na. Cl • Example 3: ▫ Make up 1 L of 5 m. M Na. H 2 PO 4

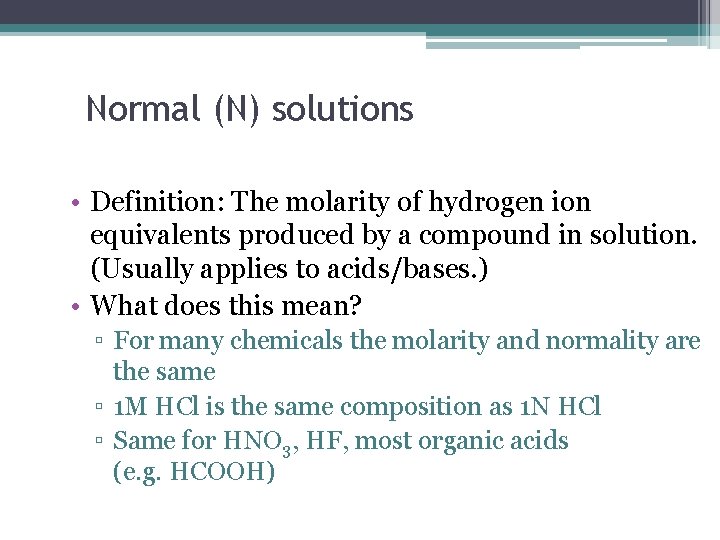
Normal (N) solutions • Definition: The molarity of hydrogen ion equivalents produced by a compound in solution. (Usually applies to acids/bases. ) • What does this mean? ▫ For many chemicals the molarity and normality are the same ▫ 1 M HCl is the same composition as 1 N HCl ▫ Same for HNO 3, HF, most organic acids (e. g. HCOOH)

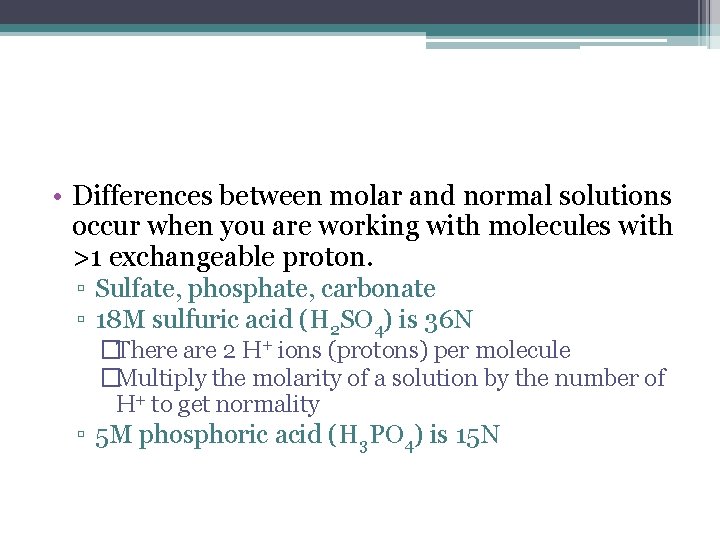
• Differences between molar and normal solutions occur when you are working with molecules with >1 exchangeable proton. ▫ Sulfate, phosphate, carbonate ▫ 18 M sulfuric acid (H 2 SO 4) is 36 N �There are 2 H+ ions (protons) per molecule �Multiply the molarity of a solution by the number of H+ to get normality ▫ 5 M phosphoric acid (H 3 PO 4) is 15 N

Dilution from one concentration to another C 1 V 1=C 2 V 2 C 1 = initial concentration V 1 = initial volume C 2 = desired final concentration V 2 = desired final volume

Examples • You need 100 ml of 1 M Na. Cl and you have a stock solution of 5 M Na. Cl ▫ What do you know? �C 1 = 5 M �C 2 = 1 M �V 2 = 100 ml ▫ Answer … solve for V 1 = C 2 V 2/C 1 ▫ = 1 M x 100 ml/5 M = 20 ml of the stock

Making very dilute solutions • When making very dilute solutions (micromolar concentrations, etc. ) you may need to design a 2 step protocol. • You can’t weigh out <10 mg easily. • So use more, make a concentrated stock solution, and then dilute from the stock.

One other complication • Sometimes a concentration is specified in terms of one element in the solution: ▫ Make up 1 L of 10 ppm Zn solution ▫ That would be 10 mg Zn per liter. ▫ But elemental Zn is an insoluble metal! • Use zinc sulfate (heptahydrate) – Zn. SO 4 ▫ You’ll need to use more than 10 mg of zinc sulfate to get the 10 mg of zinc that you want. • Multiply amount of zinc needed by the ratio of formula weight to atomic weight of Zn.

Special considerations for strong acids and bases • SAFETY: Whenever possible, add acid to water, not water to acid. ▫ Important primarily for very concentrated acids ▫ Prevents splashing & over-heating of acid • “Full-Strength” is not necessarily 100%. ▫ Ex. : 100% HCl is a gas! The most concentrated liquid form is a 36% aqueous solution. ▫ So when diluting to make 10% HCl, you are starting from 36%, not 100%. ▫ Acid concentration chart

Practice problems • 1. Describe how you would make up ▫ 250 ml of 200 m. M Na. Cl. ▫ 250 ml of a 0. 05 M glucose solution ▫ 10 ml of 10 -5 M glucose solution starting from a 0. 05 M stock solution • 2. How many grams of acetic acid would you use to make 10 L of a 0. 1 M solution of acetic acid?

• 3. What volume of 10 M acetic acid is required to produce 1 liter of 0. 5 M acetic acid? • 4. Describe how to make a 2 N solution of sulfuric acid.

• 5. Describe how to prepare 500 ml of a 0. 25 M solution of sodium hydroxide from a 1. 0 M stock solution of sodium hydroxide.

More Practice Problems For hints and answers to these problems go to: http: //facweb. furman. edu/~apollard/bio 22/solution_problems. htm 1. Outline how you would prepare 250 ml of a 500 m. M solution of potassium chloride. For this problem only, and not for those that follow, give complete details of what glassware you would use, and the mechanics of making the solution. 2. How would you prepare 100 ml of a 5 µM solution of ammonium nitrate (NH 4 NO 3)? 3. Pete Paladin measures out 30 ml of 1 M sucrose solution, and then adds water to make a total volume of one liter. What is the molarity of the final solution? 4. Outline how you would prepare 1 liter of 10% lactic acid, from a bottle you would probably find in the laboratory supply cabinet. What extra step should you take as a safety precaution? 5. Outline how you would prepare one liter of a solution of 5 ppm nickel. The chemical available to you is nickel chloride (Ni. Cl 2· 6 H 20). 6. What is the molarity of nickel in the 5 ppm solution prepared above?
 Water and water and water water
Water and water and water water Example of a news story
Example of a news story Inverted pyramid in news writing
Inverted pyramid in news writing Least important to most important
Least important to most important Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worms-breton
Tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn