WarmUp Property Any characteristic that allows us to























- Slides: 39

Warm-Up Property: Any characteristic that allows us to recognize a particular type of matter and distinguish it from other types of matter. Why do you think it is important for matter to have properties and for us to know what those properties are?

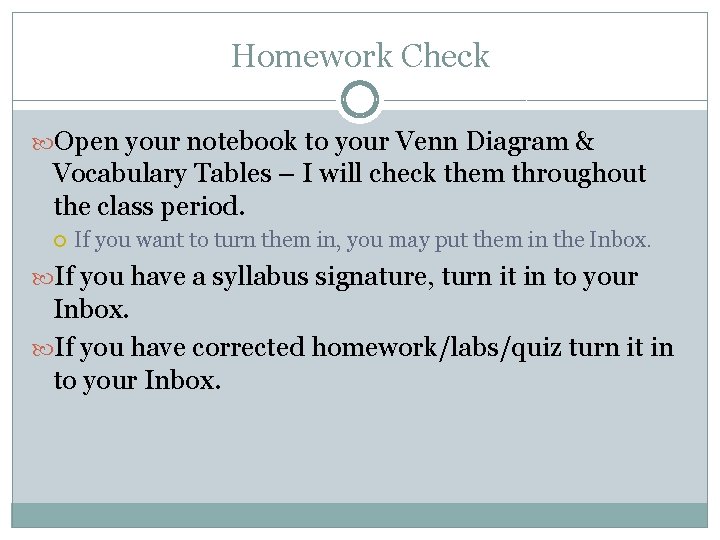
Homework Check Open your notebook to your Venn Diagram & Vocabulary Tables – I will check them throughout the class period. If you want to turn them in, you may put them in the Inbox. If you have a syllabus signature, turn it in to your Inbox. If you have corrected homework/labs/quiz turn it in to your Inbox.

Objective Distinguish between physical and chemical properties and changes.

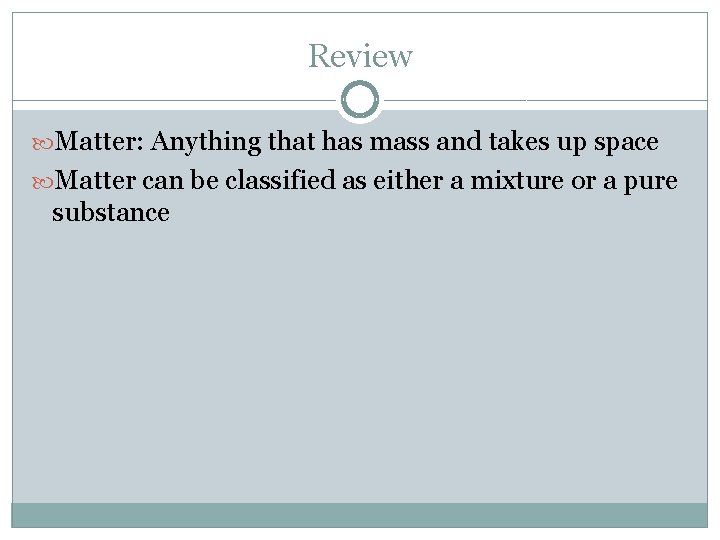
Review Matter: Anything that has mass and takes up space Matter can be classified as either a mixture or a pure substance

Types of Matter Substance - matter that is either an element or a compound. Cannot be separated by physical means. Mixture - a material made of elements or compounds mixed together, but not chemically combined. Can be separated by physical means.

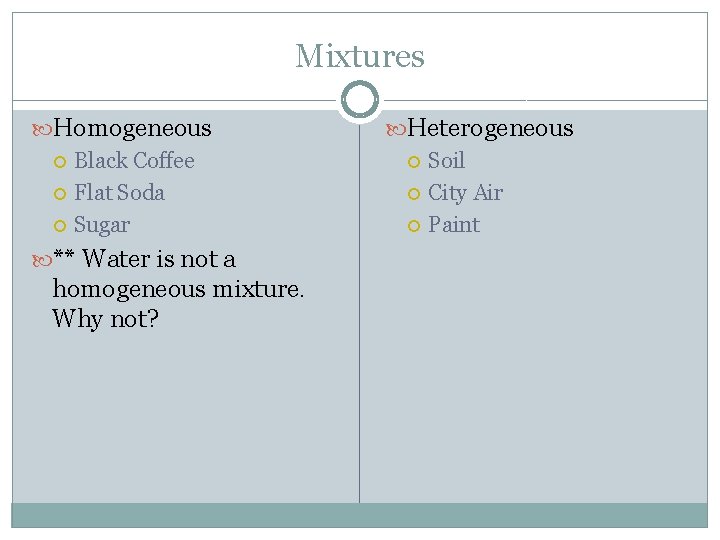
Mixtures Homogeneous Black Coffee Flat Soda Sugar ** Water is not a homogeneous mixture. Why not? Heterogeneous Soil City Air Paint

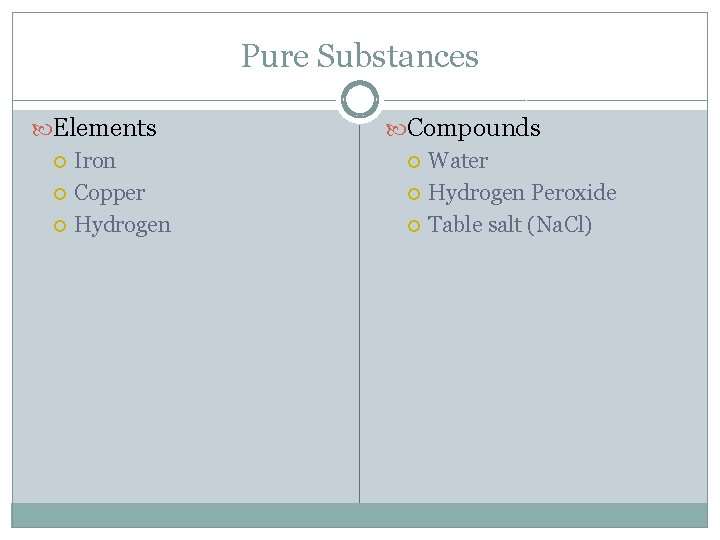
Pure Substances Elements Iron Copper Hydrogen Compounds Water Hydrogen Peroxide Table salt (Na. Cl)

Properties of Matter

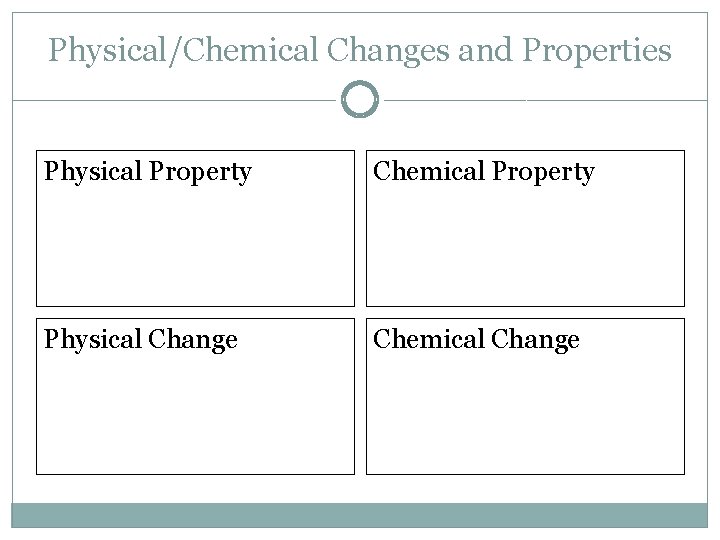
Physical/Chemical Changes and Properties Physical Property Chemical Property Physical Change Chemical Change

Properties A property is any characteristic that allows us to recognize a particular type of matter and distinguish it from other types.

Physical Property Can be observed without changing the composition of the substance.

Examples of Physical Properties • Color, shape, phase, texture, hardness, density, boiling point, melting point and odor.

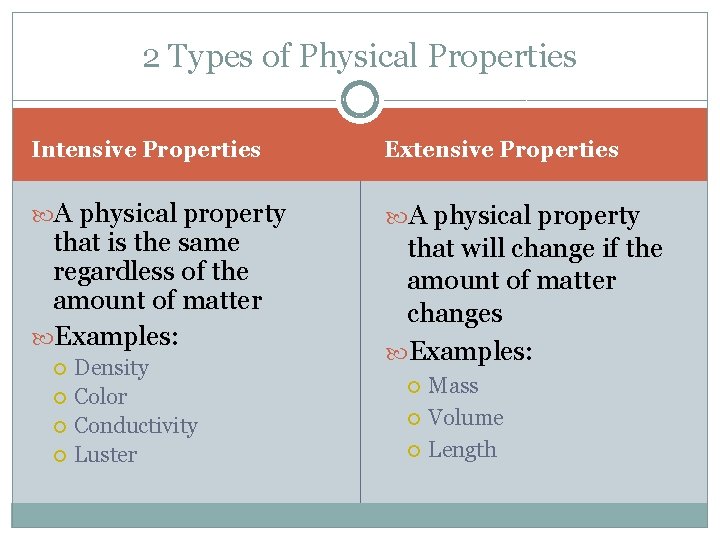
2 Types of Physical Properties Intensive Properties Extensive Properties A physical property that is the same regardless of the amount of matter Examples: Density Color Conductivity Luster that will change if the amount of matter changes Examples: Mass Volume Length

Chemical Property Can only be observed by changing the composition of the substance.

Examples of Chemical Properties Acidity, p. H, combustibility, and reactivity.

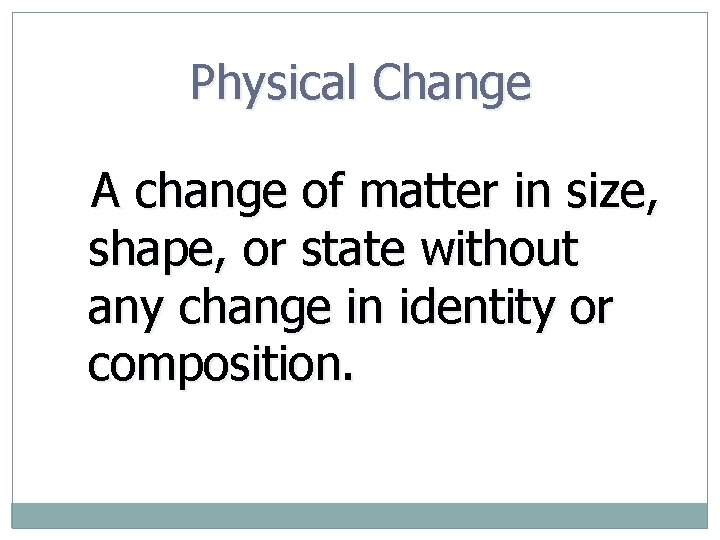
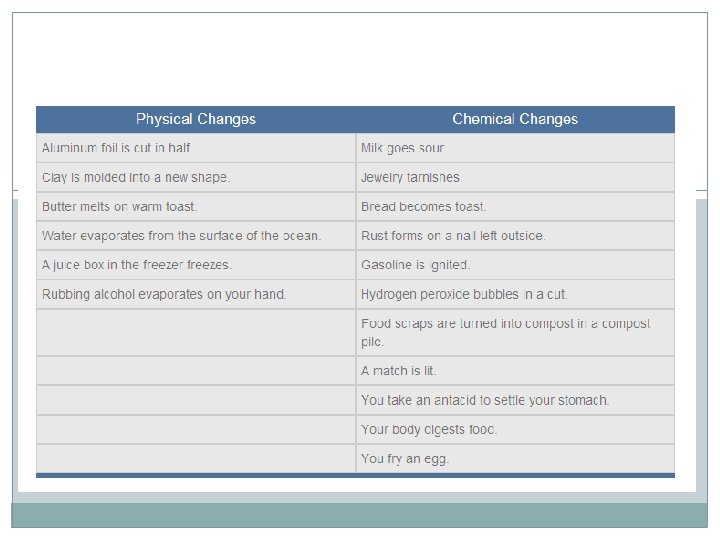
Physical Change A change of matter in size, shape, or state without any change in identity or composition.

In other words……… • A physical change does not change what the substance is or what it is made of.

Chemical Change • The change of a substance into a new and different substance. • NEW SUBSTANCES ARE FORMED • Also known as a chemical reaction

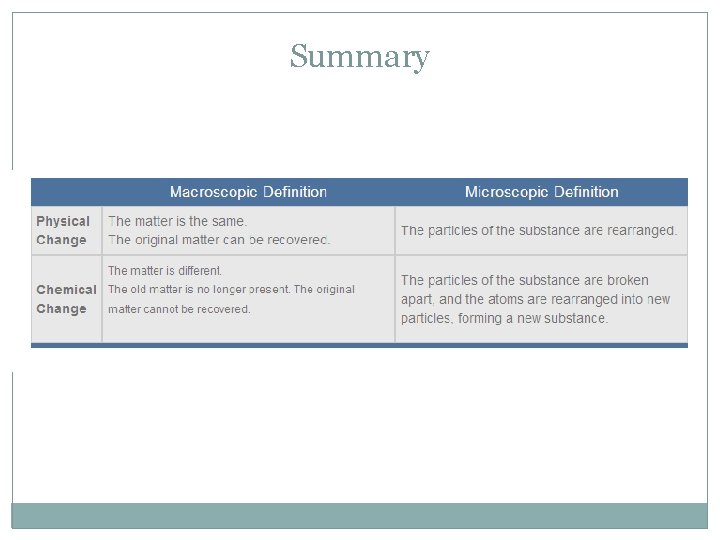
Summary


Choose a Corner For the following examples, you will decide whether the change is Physical or Chemical YOU MUST WALK TO THE CORNER THAT REPRESENTS YOUR ANSWER Be prepared to support your answer

Dissolving sugar in water PHYSICAL

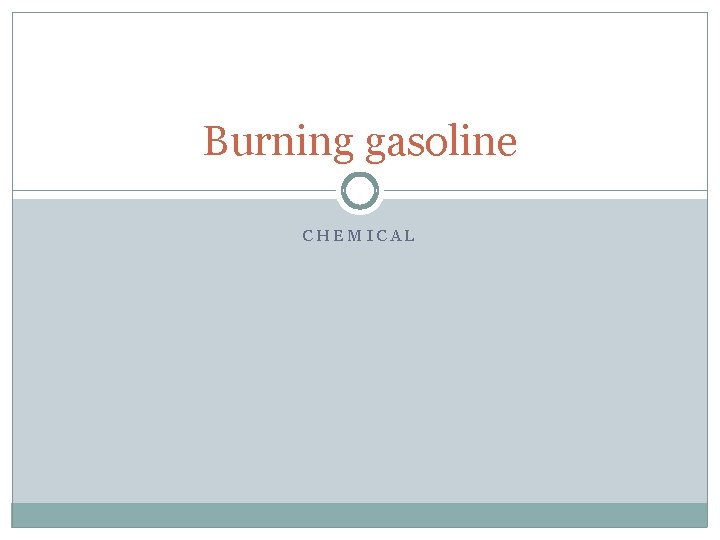
Burning gasoline CHEMICAL

Bread Rising CHEMICAL

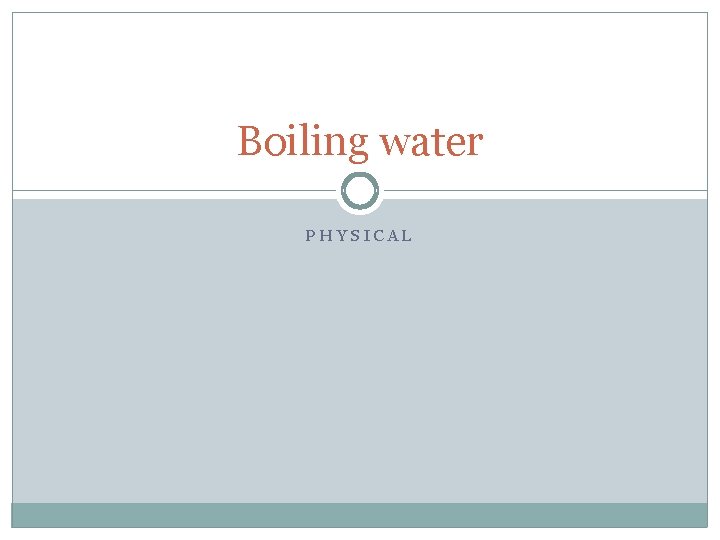
Boiling water PHYSICAL

Sun Tanning CHEMICAL

Whipping Egg Whites PHYSICAL

Magnetizing a compass needle PHYSICAL

Milk souring CHEMICAL

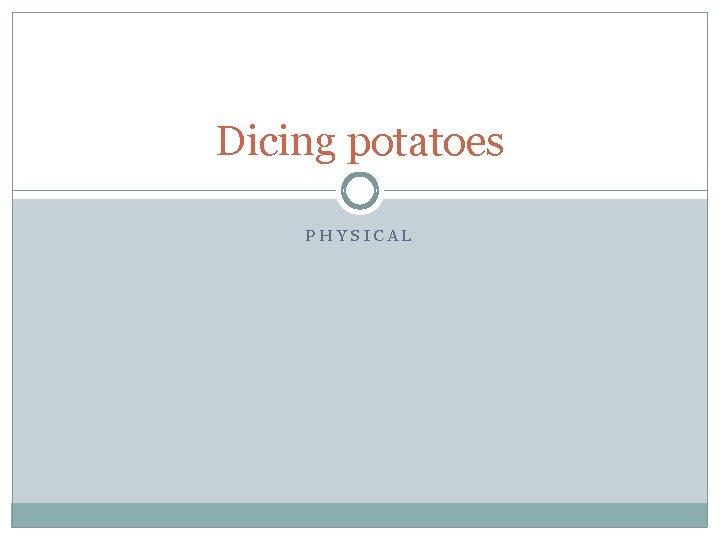
Dicing potatoes PHYSICAL

Dissolving Alka-Seltzer in water CHEMICAL

Cooking eggs CHEMICAL

Digestion CHEMICAL

Jewelry Tarnishing CHEMICAL

Rubbing alcohol evaporates PHYSICAL

Lighting a match CHEMICAL

Closure Before you leave…list 1 thing you learned today 1 question you still have about physical and chemical properties and/or physical and chemical changes Homework: Due Sept 6 th Finish the worksheet for physical vs chemical properties Finish the worksheet for physical vs chemical changes

Poster Define: Chemical Property Physical Property Chemical Change Physical Change Give an example of each one Can be an image or write the word
