The Properties of Matter Characteristic vs Non characteristic














- Slides: 15

The Properties of Matter

Characteristic vs. Non characteristic properties A characteristic property is something that is specific to that substance. (i. e. Density, melting point, boiling point) Non-characteristic properties are common to many substances. (i. e. states of matter, temperature, volume, mass, acidity and alkalinity)

Brainstorm With a partner write down 2 examples of characteristic properties of a substance and/or material. And. . . 4 examples of non-characteristic properties I will give you 10 minutes to write down your answers and then I will randomly pick a few pairs to discuss their answers.

Terminology States of matter : (solid, liquid or gas) Mass : the quantity of matter of the substance Volume: the space that matter occupies Temperature: quantity of heat an object or matter contains Acidity and alkalinity : determines whether a substance is an acid, a base or neutral in its chemical composition.

Properties of matter

States of Matter Solids: - Particles held tightly together by chemical bonds - Particles cannot move freely. Only vibrate

States of Matter Liquids: - Particles held weakly together (compared to solids) - Particles can move slightly with respect to each other.

States of Matter Gases: - Particles are far apart from each other. - Particles move freely

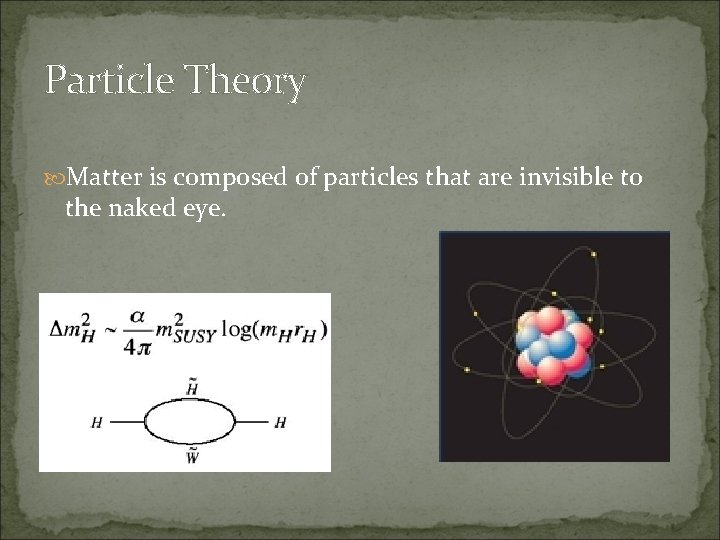
Particle Theory Matter is composed of particles that are invisible to the naked eye.

UNITS Metre : (m) Length Kilogram : (kg) mass Gram : (g) mass Second: (s) time Litre (L) volume Millilitre (m. L) volume Cubic centimetre cm 3

Volume Definition: the magnitude of the three-dimensional space enclosed within or occupied by an object, geometric solid, etc.


Brainstorm With a partner brainstorm 5 reasons of why it is important to measure the volume of various substances and/or materials. I will give you 10 minutes to write down your 5 reasons then I will pick randomly a few pairs to discuss their answers.

LAB TOUR! Follow me!

SCIENCE!
 What is a non characteristic
What is a non characteristic Non characteristic properties examples
Non characteristic properties examples Toc toc toc quelqu'un frappe à ma porte
Toc toc toc quelqu'un frappe à ma porte Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Grey matter
Grey matter Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Median and lateral apertures
Median and lateral apertures Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter White matter vs grey matter
White matter vs grey matter Flow energy review
Flow energy review Detail the measurable properties for all waves
Detail the measurable properties for all waves Liquid state of matter properties
Liquid state of matter properties Properties of matter vocabulary
Properties of matter vocabulary Composition of matter concept map
Composition of matter concept map