Viral haemorrhagic fevers VHF Prof Dr Maha AlNuaimi














































- Slides: 54

Viral haemorrhagic fevers (VHF) Prof. Dr. Maha Al-Nuaimi (C M)

Objectives • What is the VHF (s). • The clinical manifestations. . • epidemiology of the commonest types of VHF.

What is VHF • VHF syndrome. • Viral hemorrhagic fever (VHF) is an acute febrile syndrome characterized by systemic involvement, which includes malaise, prostration, generalized bleeding in severe infections, caused by several distinct families of viruses.

Clinical Features: • Initial nonspecific prodromal stage: Fever, Malaise, Headache , Myalgia/ arthralgia, Abdominal pain, Non-bloody diarrhea • Clinical multi-system illness associated with fever & bleeding diathesis. • Then Progresses to more severe symptoms & death. ü Many of these viruses cause severe life-threatening dis. ü some types of hemorrhagic fever viruses can cause relatively mild illnesses. ü The clinical manifestations depend on ? ?

Does bleeding always occur? ? Does bleeding in VHF is always life threatening? ? • Index of disease severity, • Multiorgan failure, • high mortality rates. • But, bleeding, does not always result in a life-threatening loss of blood volume.

ARBOVIRUSES (arthropod-borne viral diseases) WHO definition • Viruses maintained in nature by biological transfer between susceptible vertebrate hosts via haematophagous arthropods • multiply and produce viraemia in vertebrates, • mutiply in tissues of arthropods


Arbo-viruses • • > 500 viruses, only 26 cause significant dx Zoonotic Infective dose? Commonest vector? ?

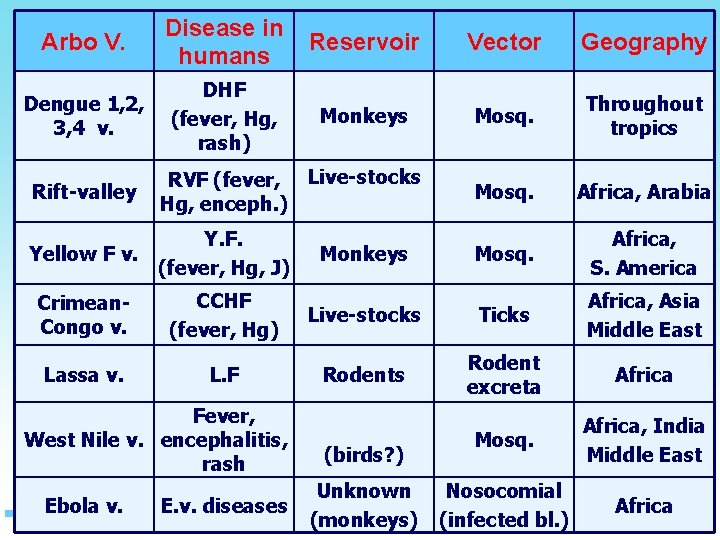
The most important VHF in the Eastern Mediterranean Region are • • • yellow fever, Rift Valley fever, dengue haemorrhagic fever, Crimean–Congo haemorrhagic fever and Ebola haemorrhagic fever

Arbo V. Disease in humans Reservoir Vector Geography Dengue 1, 2, 3, 4 v. DHF (fever, Hg, rash) Monkeys Mosq. Throughout tropics Mosq. Africa, Arabia Mosq. Africa, S. America Rift-valley RVF (fever, Live-stocks Hg, enceph. ) Y. F. Yellow F v. (fever, Hg, J) Monkeys Crimean. Congo v. CCHF (fever, Hg) Live-stocks Ticks Africa, Asia Middle East Lassa v. L. F Rodents Rodent excreta Africa Mosq. Africa, India Middle East Nosocomial (infected bl. ) Africa Fever, West Nile v. encephalitis, rash Ebola v. E. v. diseases (birds? ) Unknown (monkeys)

How is haemorrhagic fever spread? • Some viral hemorrhagic fevers are spread by mosquito or tick bites. • Others are transmitted by contact with infected blood or tissues. • A few varieties can be inhaled from infected rat feces or urine.

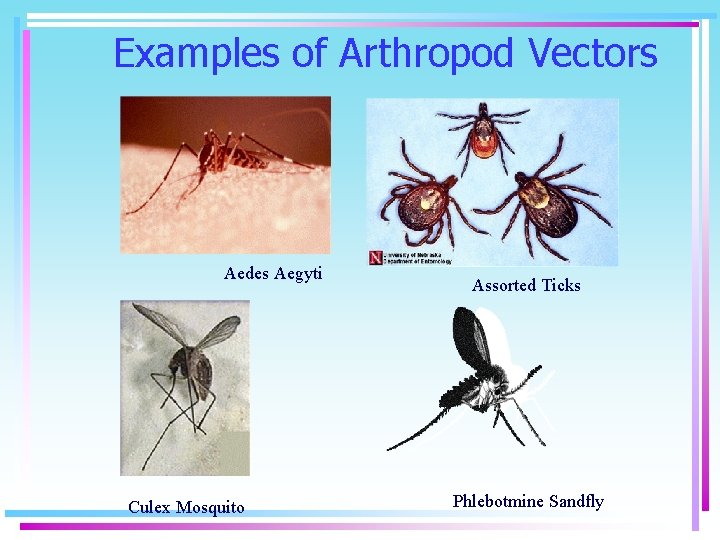
Examples of Arthropod Vectors Aedes Aegyti Culex Mosquito Assorted Ticks Phlebotmine Sandfly

prolonged outbreaks of VHF is expected in EMR. . why? ? • Lack of timely laboratory diagnosis • Lack of Functional epidemiological surveillance, • Inadequate infection control practices at health care facilities • Weak vector control programs.

DIAGNOSIS • Positive antigens and antibodies • Isolation of the virus from the body.

Transmission of haemorrhagic fever viruses • The arthropod vectors are spread most often when the vector mosquito or tick bites a human, or when a human crushes a tick. • By dealing or slaughtering the infected animals, livestock. • The viruses carried in rodent reservoirs are transmitted when humans have contact with urine, fecal matter, saliva, or other body excretions from infected rodents. • Person to person transmission, e. g Ebola, Marburg, Lassa and Crimean–Congo haemorrhagic fever viruses. (direct contact or their body fluids • Contaminated objects. As, contaminated syringes and needles. . Ebola haemorrhagic fever and Lassa fever.

Treatment • No specific treatment. • Ribavirin, which has been effective in treating Lassa fever. • Supportive treatment: IVF, Bd, oxygene and the alleviation of symptoms. As restlessness, confusion, myalgia, and hyperesthesia; should be managed by reassurance and some drugs as sedatives, pain relief, and amnestic medications. • Pulmonary insufficiency may develop, • yellow fever, hepatorenal syndrome is prominent. • Treatment of Bleeding. Fresh frozen plasma, clotting factor concentrates, and platelets, with the use of heparin for prophylaxis of DIC. ü Survival depends largely upon the virulence of the virus strain and the quality of treatment.

Prognosis • Recovery from some VHF. • Fatality rates of VHF Ranging from 5% to >20%. (Dengue HF 5 -15%, Ebola and Marburg outbreaks 25% to 90%) • Sporadic has less fatality than epidemic cases. yellow fever; 5% of sporadic are fatal, 20%– 50% of epidemic cases may be fatal. • Permanent disability: 10% of severely ill Rift Valley fever suffer retina damage and may be permanently blind, and 25% of South American haemorrhagic fever suffer permanent deafness. ü Immunity ? ? ü Lifelong immunity against the virus that made them ill.

Prevention of HF • Haemorrhagic fevers can be prevented through vector control and personal protection measures. • Attempts have been made in urban and settled areas to destroy mosquito and rodent populations. In areas • where such measures are impossible, individuals can use insect repellents, mosquito netting, and other • methods to minimize exposure. Vaccines have been developed against yellow fever, Argentinean • haemorrhagic fever and Crimean–Congo haemorrhagic fever. Vaccines against other haemorrhagic fevers • are being researched • The only established and licensed virus-specific vaccine available against any of the HF viruses is the yellow fever live attenuated 17 D vaccine, which is mandatory for travelers to endemic areas of Africa and South America

Isolation • Virus in their bd and even other screations. So, caution is needed when evaluating and treating patients with suspected VHF syndrome. Overreaction by medical personnel is inappropriate, but it is prudent to provide isolation measures as rigorous as feasible. At a minimum, isolation measures should include the following: • • Restricted access to the patient and use of stringent barrier nursing including mask, gown, glove, and needle precautions. • Proper hazard labeling of all specimens submitted to the clinical laboratory with notification of appropriate clinical personnel. • Proper disposal of all material within the isolation room by autoclaving or liberal disinfection of contaminated materials using such disinfectants as hypochlorite or phenols. • For more intensive care, however, increased precautions are recommended. Members of the patient-care team should be limited to a small number of selected, trained individuals, and special care should be directed toward eliminating all parenteral exposures. Use of endoscopy, respirators, arterial catheters, routine blood sampling, and extensive laboratory analysis increases opportunities for aerosol dissemination of infectious blood and body fluids. For medical personnel, wearing flexible plastic hoods equipped with battery-powered blowers provides excellent protection of the mucous membranes and airways.

• VIROLOGY OF VHF (FEATURES OF THE VIRUSES) • All are single-stranded RNA. • All are enveloped (transmission by foods or drinks? ? ? ). • Infectious during viremia stage. • Low infectivity dose (1 -10 viruses can cause infections). • Geographically restricted to areas where host lives. • Humans are not the natural reservoir but accidentally infected when comes in contact with infected hosts. • Human outbreaks are sporadic and irregular. • 7. • When human is infected can infect another human • No established treatment (with few exceptions) • The best treatment is control of infection. • Role in bioterrorism (biological weapons due to high morbidity and mortality and due to aerosol transmission of most of them except dengue fever virus)

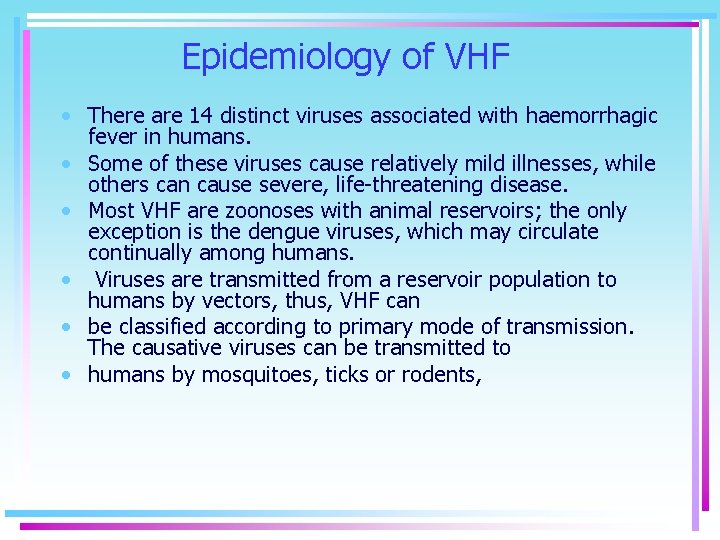
Epidemiology of VHF • There are 14 distinct viruses associated with haemorrhagic fever in humans. • Some of these viruses cause relatively mild illnesses, while others can cause severe, life-threatening disease. • Most VHF are zoonoses with animal reservoirs; the only exception is the dengue viruses, which may circulate continually among humans. • Viruses are transmitted from a reservoir population to humans by vectors, thus, VHF can • be classified according to primary mode of transmission. The causative viruses can be transmitted to • humans by mosquitoes, ticks or rodents,

• • • EPIDEMIOLOGY OF HFV DISEASE TRANSMISSION - HFVs are zoonosis: Animal hosts (Rodents) and arthropod vectors are main reservoirs. A. Natural infection of humans (mode of transmission): 1. Bite of infected arthropod (ticks or mosquitos) 2. Aerosol from infected rodent excreta 3. Direct contact with infected animals/carcasses or fomites B- Human to Human & Nosocomial Transmission • Possible for most HFVs (except ? ? ) - Most person-to-person spread due to direct contact with infected blood & body fluids (Hospital acquires infections, HAIs - Mucous membrane contact, - Aerosolized, (airborne in Ebola, Lassa, Junín, & may be yellow fever) - Semen, - vomitus - Sweat, • Incubation period ranging from 2 to 21 days for all of them. 10. PATHOGENESIS • The target organ is the vascular bed (hemorrhage) • The replication of virus is intracellularly • Cytokine release leads to shock and hypotension. • Affects platelet functions and numbers (thrombocytopenia) • Affects bone marrow and clotting factors. 11. CASE-FATALITY (MORTALITY) RATE Virus Mortality rate Filoviruses Around 90% for Ebola virus (the highest) Flaviviruse s 0. 5% Arenavirus es 15 -30% Causes of death: 1 - Hemorrhagic diathesis (several body sites & orifices) 2 - Shock 3 - Multiorgan failure

• Case fatality rate (CFR) approaches 90%. • The virus was transmitted to humans from wild animals. • Fruit Bats are considered as the natural host for the virus. • Other reservoirs rodents and plant virus?

EBOLA VIRUS DISEASE TRANSMISSION 1 - By direct . 1 human-to-human contact with the blood, or other body secretions (saliva, breast milk, tears, stool, skin, or semen) of infected persons or even Dead bodies. (N. B. Lab staff at risk) 2 - Transmission through semen may occur up to 7 weeks after clinical recovery (Sexual transmission). 3 - Indirect contact transmission via environment contaminated with such infected secretions. 4 - By handling ill or dead infected chimpanzees or other infected animals. 16. 5 - Health care workers (HCWs) have frequently been . 2 infected while attending patients (direct contact body fluids, Needle sticks, aerosols). 17. • Health care workers (HCWs) have critica. 3




• • • Filoviruses: Ebola & Marburg - Cause severe HF that similar to fulminant septic shock. - Mortality Rate: Ebola: 50 -90% Marburg: 2530% - Incubation period: 2 -21 days for Ebola (Mean 8 -10). 3 -10 days for Marburg 9. 6 days (mean) from symptom onset to death 19. VIROLOGY • Filovirus family includes Ebola and Marburg viruses. • Single stranded-RNA (ss. RNA), enveloped virus. • Ebola virus contains 5 strains with different phylogenic tree (Zaire, Sudan, Ivory Coast, Reston & New 5 th Guinea strain. • High mutational potentials. • Rapidly replicating within 8 Hs. • U or 6 -shape virus. 20. What is wrong here? ? 21. II- Flaviviruses A- Yellow Fever B- Dengue Fever C- Omsk HF D- Kyasanur Forest Disease N. B. Flavus in Latin means yellow. 22. Mode of transmission - Yellow Fever – Aedes mosquito (A. aegypti, A. africanus, A. simpsoni, A. furcifer, A. luteocephalus, and A. albopictus (Asian tiger mosquito) - Dengue Fever – mosquito (Aedes aegypti) - Omsk HF/Kyasanur FD: Tick bite No reported cases of person to-person transmission 23. Yellow Fever cycles 3 cycles for yellow fever: Jungle Urban intermediate

Flavivirus. 1 25. A- Yellow Fever Incubation period: short - 3 -6 days. C/P: 1 - Initial symptoms . 2 Fever, chills, severe HA, back pain, muscle aches, nausea, fatigue. Most symptomatic patients develop only this stage however, in 15% of symptomatic patients severe form will develop after short period of symptom remission (Toxic shock). 2 - Toxic phase - fever returns with initial symptoms PLUS Coagulopathy & hemorrhage - hematemesis (black vomit) Jaundice, Hypotension, shock, metabolic acidosis, arrhythmias Confusion, seizures, and coma can occur. 26. N. B. The majority of persons infected with yellow fever virus have no illness or . 3 only mild illness. - Faget’s sign – relative bradycardia with fever. - Mortality rate: 510% (20 -50% in epidemics and hospitalized pts). - N. B. 90% of cases occur in Africa, 10% in South America. Vaccine is available (Atiqa PHC & Airport) and indicated to travels to endemic area in Africa or South America. - live-attenuated vaccine, single dose, - To > 9 months travelers or living in endemic areas - Immunity in 1 week in 95% of people - Protection for 30 -35 years. - No specific treatment available. 27. 90% of yellow fever cases occur in Africa. 4 28. 10% of yellow fever cases occur in S. America. 5 29. - Described as “breakbone fever” by Benjamin Rush in 1789. - Endemic . 6 throughout Americas, Asia & Africa - Vector - Aedes aegypti & Aedes albopictus. - Virus replicates in mosquitos. - Very Rare by blood transfusion, organs transplant. & Vertical transmission). - Incubation period: 3 -14 days. - The Most prevalent mosquitoborne viral disease in the world. - 1/3 of world populations are exposed (400 million cases yearly) B- Dengue Fever




• > 100 countries have endemic dengue transmission. - In USA: Dengue - 10. 4% of post-travel systemic febrile illness for travelers returning from endemic areas. - No vaccine available (in 2015 new one is used successfully by Sanofi Pasteur for people in endemic areas). - No specific treatment. - In KSA it is present in Mecca and Jeddah.



• • • Four Dengue Virus Serotypes (DEN 1, 2, 3, and 4) - All can cause severe & fatal infection - Infection by one serotype gives No cross immunity to other types but life long immunity to the same type, however, more predisposition to DHF/DSS if infected by another serotype. - 2 o immunopathological mechanism triggered by sequential infections with different dengue viral serotypes. - Complicated pathogenesis – partially attributable to Ab- dependent enhancement. - Humans are the main reservoir but monkeys may be. 32. 4 Clinical Manifestations of disease 1 - Undifferentiated fever 2 - Classic Dengue Fever 3 - Dengue Hemorrhagic Fever 4 - Dengue Shock Syndrome 33. 2 - Classic Dengue Fever - Acute febrile illness - Severe Hemorrhage mainly retroocular; - Myalgia & arthralgia – often severe (breakbone fever); - Nausea & vomiting > 50%; diarrhea (30%) - Rash (50%) (of variable appearances; maculopapular, petechial, or erythematous. 34. 3 - Dengue Hemorrhagic Fever (DHF): - Most serious form of dengue infection - WHO estimates 500, 000 cases /year - Mortality ≈ 10%; high as 50% - WHO 4 diagnostic criteria (Fever (2 -7 days) – Hemorrhagic manifestations – Low platelet counts (< 100000 /ml) – evidence of leaky capillaries) 35. 4 - Dengue Shock Syndrome (DSS): 4 WHO criteria for DHF AND Evidence of circulatory failure or shock: - Rapid, weak pulse, narrow pulse pressure (< 20 mm Hg) - Hypotension for age - Cold, clammy skin, AMS (Altered Mental Status

A- Rift Valley Fever - Mosquito-borne disease affects primarily sheep & goats also cattle, buffalos and camels. - Most human infections are unapparent - Self limited febrile illness first reported in Kenya’s Rift Valley in the early 1910 s. - Rare severe forms (Ocular; retina, Meningoencephalitis, or Hemorrhagic fever form) - 1% develops typical VHF. - Short incubation: 3 -6 days. - Mortality (variable) but less than 1%. 39. Humans acquire infection by: - Bite of infected mosquito (several species- vertical trans for yrs) - Contact with infected animal tissues - Aerosolization of virus from infected animal carcasses (Lab Staff) - Ingestion of contaminated raw animal milk? ? ? (No reported cases of human-to-human transmission- still theoretical risk to HCWs). - Standard precautions are enough. - Vaccination of animals but not during epidemics. - Inactivated human vaccine is not licensed for use. 40. KSA Outbreak * - Saudi Arabia, Jizan & Asir (from 26 August 2000 through 22 September 2001) 886 infected / 87 dead (13. 7%). - The first time infection to be reported out of Africa. - Vision loss 10/683. - Hemorrhagic manifestations were in 35/494. - CNS and liver affection occurs. * http: //www. kau. edu. sa/Files/140/Researches/50678_20846. pdf 41. Rift Valley fever Distribution • •

• • • DIAGNOSIS, MANAGEMENT AND CONTROL OF VHF 47. Diagnosis of VHF & HFVs Case Definition, VHFs – Ebola, Marburg, New World Arenaviruses, Old World Arenaviruses, and CCHF (CDC 2011). Patient must have: One or more of the following - Fever ≥ 40 o. C. - No predisposing factors for hemorrhagic manifestations -AND no established alternative diagnosis -Severe headache -Muscle pain -Erythematous maculopapular rash on the trunk with fine desquamation 3– 4 days after rash onset - Vomiting -Diarrhea - Abdominal pain - Thrombocytopenia - Bleeding not related to injury - Pharyngitis (arenavirus only) - Retrosternal chest pain (arenavirus only) 48. Important History within 21 days of: 1. Patient from or travel to endemic areas �� Even if nonspecific S+S �� Comprehensive travel history critical 2. History of tick, mosquito bites. 3. Contact with mice or their excreta 4. History of contact with patient with above risk factors & VHF symptoms 5. Contact with sick animals or carcasses in endemic areas. 6 - In the event of Bioterrorist attack event 49. 1 - Non-specific Lab Abnormalities - Leukopenia -Anemia - Hemoconcentration - Thrombocytopenia - Elevated LFTs -Azotemia 2 - Coagulation abnormalities - Prolonged bleeding time, PTT - Increased FDP (fibrin degradation products) - Decreased Fibrinogen - DIC A- Non specific Lab Abnormalities in HFV Infection 50. Test Notes Lab level Antigen detection by PCR - The Early rapid diagnostic test 2 nd or 3 rd BSL Antigen detection by ELISA test Rapid diagnostic test. 2 nd or 3 rd BSL Ig. M detection by ELISA Late diagnosis after 10 days of onset of infection 2 nd or 3 rd BSL Viral isolation Takes 3 -days to complete. Mostly in research less in 4 th BSL B- Specific Lab diagnostic test in HFV Infection

• • Supportive (Main treatment) Specific antiviral treatment Contraindicated Isolation (Airborne in Lassa, Ebola Marburg) No FDA approved antiviral agents. Aspirin & NSAIDs Fluid & electrolyte balance Ribavirin used in Arenaviruses and in Bunyaviruses. Anticoagulant therapies Supplemental O 2, & Mechanical Ventilation Ribavirin not active against (F) Filoviruses of Flaviviruses Steroids are of no benefit Treatment of HFVs Infection 52. Supportive (Main treatment) Specific antiviral treatment Contraindicat ed Circulatory & BP support Early vasopressors Blood products: (Platelets Clotting factor, & FFP Pain control Secondary infections common -aggressive treatment with Treatment of HFV Infection (Continue) 53. Immunization and infection control in HFVs 1 - Passive immunization • Immune plasma in Arenavirusis (Junin and Machupo) • Whole blood from Ebloa survivors 54. 2 - Active Immunization for HFVs 1 - Live-attenuated vaccine available and effective for yellow fever virus. 2 - Inactivated virus for dengue fever is under trial with good success 3 - Vaccines for Junin (Argentine) virus under trial. 4 - Also Rift Valley virus vaccine is under trial.

• • • INFECTION CONTROL & HFVS Infection-control Guidelines: - Stick to the standard precautions is the gold standard. - Report suspected cases immediately to Infection control officer, MOH, which notify the CDC, - Isolation of all suspected cases (Negative pressure Room or single room) - Strict hand washing (HH) - Double gloving - Use of impermeable gowns 56. - N-95 masks or powered air-purifying respirators (PAPR). - Negative pressure isolation with 612 air exchanges / hour - Leg & shoe covering - Face shields & goggles - All contacts of patients diagnosed with VHF including hospital personnel & lab workers should be placed under medical surveillance for signs of VHF infection for 21 days. 57. NOTIFICATION PROCESS IN VHF (MOH-KSA) • The following notifications are mandatory if suspected cases of VHF are admitted: 1. The Admitting Consultant notifies the: a. Infectious Diseases Consultant. b. Nurse in charge of Emergency Department and Ward where patient is to be admitted. 2. The Infectious Diseases Consultant notifies the: a. Chairman of the Infection Control Committee who will then notify the: i. Medical Director ii. Executive on Duty 58. 3. The Nurse in charge of ERnotifies the: a. Nursing Supervisor or Duty Administrator b. ICU Head Nurse if the patient is to be admitted to the ICU 4. The Chairman of Infection Control Committee notifies the: a. Hospital Director b. Laboratory and Radiology Departments c. Employee Health Department 59. 5. The Infection Control Practitioner notifies the: a. Housekeeping Manager b. CSSD Manager c. Ministry of Health d. Utilities and Maintenance for ventilation modification in patient rooms. 6. The Nursing Supervisor notifies the: a. Director of Nursing b. Nurse manager to consult on staffing. c. Materials department for equipment for strict isolation.

• • INFECTION CONTROL AND LAB TESTING • All HFVs are highly infectious in lab setting • May be transmitted to lab personnel by small particle aerosols • Notify lab immediately if VHF suspected • All specimens should be: : - Clearly identified - Double bagged - Hand carried to lab - Do NOT transport specimens in pneumatic tube 61. CDC Recommendations for personal protection during specimen collection: • Full face shield or goggles, masks to cover all of nose and mouth, gloves, fluid resistant or impermeable gowns. Additional PPE may be required in certain situations. 62. Specimen Handling for Routine Laboratory Testing (suspected Ebola case but not for Ebola Diagnosis) (CDC) • Routine laboratory testing includes traditional chemistry, hematology, and other laboratory testing used to support and treat patients. • The following precautions are required to deal with specimens suspected to contain Ebola virus for routine laboratory: • Full face shield or goggles, masks to cover all of nose and mouth, gloves, fluid resistant or impermeable gowns AND use of a certified class II Biosafety cabinet or plexiglass splash guard, as well as manufacturer-installed safety features for instruments. 63. POST-EXPOSURE PROPHYLAXIS & MANAGEMENT • No vaccine or antiviral agents • Percutaneous or mucocutaneous exposures: Immediately wash affected skin with soap and water ; flush eyes. • High Risk Exposures & Close Contacts: - Place under medical surveillance - Record temps. 2 times /day. Report any temp. ≥ 38 o. C. Report any S/S of VHF - Initiate Ribavirin if S/S of VHF develop (Arenavirus or Bunyavirus only).

• Where is hemorrhagic fever found? Ebola and Marburg haemorrhagic fevers and Lassa fever occur in parts of sub-Saharan Africa. CCHF occurs in the steppe regions of central Asia and in central Europe, as well as in tropical and southern Africa. RVF occurs in Africa and has recently spread to Saudi Arabia and Yemen. (Maps can be found on WHO website. ) • What are the signs and symptoms of dengue hemorrhagic fever? Signs and symptoms of dengue hemorrhagic fever or severe dengue — a life-threatening emergency — include: • Severe abdominal pain. • Persistent vomiting. • Bleeding from your gums or nose. • Blood in your urine, stools or vomit. • Bleeding under the skin, which might look like bruising. • Difficult or rapid breathing.

n Because the disease is easily transmitted, Nurses must wear protective clothing when interacting with a patient and must dispose of all needles and other medical supplies properly. Ebola HF Nurses in full protective gear in Gabon, 2002

The Rift Valley RVF is an arthropod-borne, acute viral disease of sheep, goats, cattle and people.

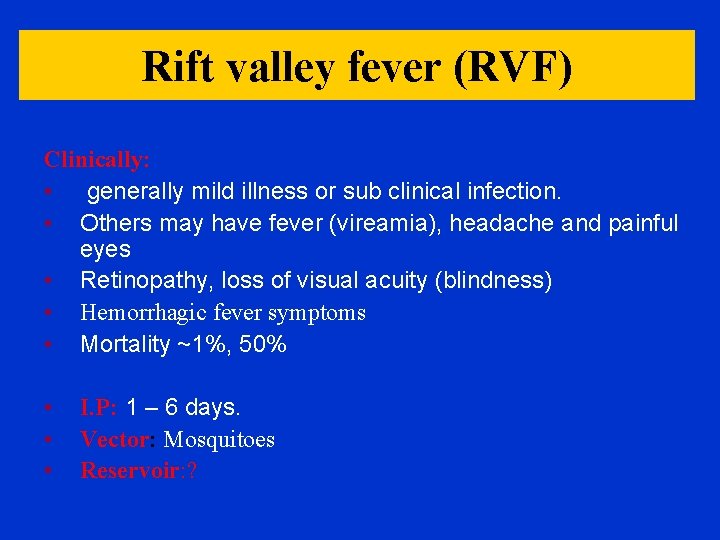
Rift valley fever (RVF) Clinically: • generally mild illness or sub clinical infection. • Others may have fever (vireamia), headache and painful eyes • Retinopathy, loss of visual acuity (blindness) • Hemorrhagic fever symptoms • Mortality ~1%, 50% • • • I. P: 1 – 6 days. Vector: Mosquitoes Reservoir: ?

Aedes furcifer

Occurrence: • • Kenya 1951 – 1982. In Sub-Saharan appeared in Egypt 1977. to Saudi Arabia and Yemen in year 2000. • Dambos & floodwater for epizootic.

Mode of transmission: p Bite of mosquitoes p handling tissues, blood, excretions of infected animals. (risky) p milk p Lab. Workers and butchers via virus containing aerosols p Noso-commial? Communicability: no direct man to man transmission

Method of control: 1. Health educate the public to protect against vector. 2. Control of mosquitoes • Environmental control. • Insecticidal, problems? • Larvacidal , biological, advantage? • Genetic methods • Integrated approaches. • Personal protection? ? ? 3. Vaccine humans → experiments Live-stock → available vaccine – No specific treatment

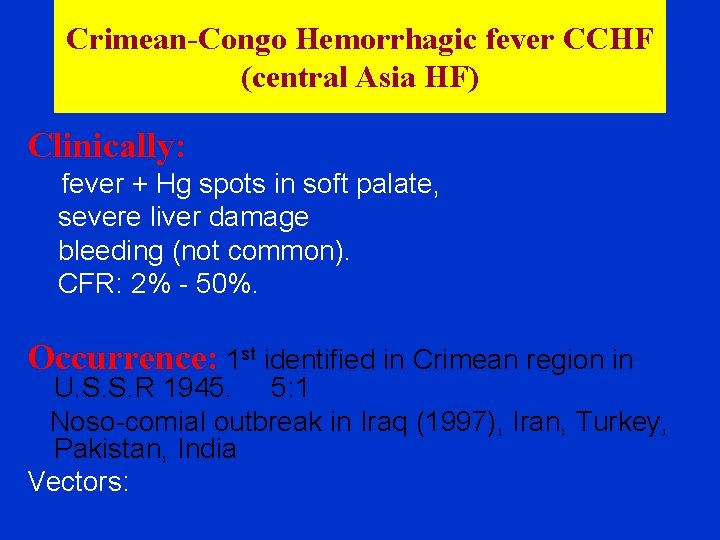
Crimean-Congo Hemorrhagic fever CCHF (central Asia HF) Clinically: fever + Hg spots in soft palate, severe liver damage bleeding (not common). CFR: 2% - 50%. Occurrence: 1 st identified in Crimean region in U. S. S. R 1945. 5: 1 Noso-comial outbreak in Iraq (1997), Iran, Turkey, Pakistan, India Vectors:


Hyalomma sp. ticks RT-PCR Viral isolation


Mode of transmission: p Bite of infective adult tick p Noso-comial infection (blood or secretion of p) p Butchering Communicability: highly infectious in hospital setting Reservoir: ? IP: 1 -3 days Method of control: 1. Health educate the public to protect against ticks. 2. Control of ticks Specific treatment → (ribavirin I. V)
 Vhf
Vhf Vhf uhf and microwave antennas
Vhf uhf and microwave antennas Sailor 6204
Sailor 6204 Vortac stands for
Vortac stands for Vhf digital link
Vhf digital link Frekuensi vhf
Frekuensi vhf Gps stands for
Gps stands for Arinc vhf coverage map
Arinc vhf coverage map Que es un vor
Que es un vor Telenor maritime dekningskart
Telenor maritime dekningskart Harrison vhf
Harrison vhf Uhfsdr
Uhfsdr Pawsey balun
Pawsey balun Frequencia vhf polícia militar sc
Frequencia vhf polícia militar sc Class of emission
Class of emission Itil wikipedia fr
Itil wikipedia fr The dynamics of viral marketing
The dynamics of viral marketing Vaccins à vecteur viral
Vaccins à vecteur viral Viral infection
Viral infection Spasmodic croup
Spasmodic croup Aerochamber definition
Aerochamber definition Viral shedding
Viral shedding An acute highly contagious viral disease
An acute highly contagious viral disease Virus dna
Virus dna Meningitis viral antibiótico
Meningitis viral antibiótico Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Vacina trplice viral
Vacina trplice viral Hgado
Hgado Viral receptors
Viral receptors Varicela variola
Varicela variola Replicação viral ciclo lítico e lisogênico
Replicação viral ciclo lítico e lisogênico Eline's viral
Eline's viral Viral life cycle
Viral life cycle Rotarix live attenuated
Rotarix live attenuated Viral
Viral Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Csf analysis interpretation
Csf analysis interpretation Viral
Viral Exocitose
Exocitose Section 24-1 viral structure and replication
Section 24-1 viral structure and replication Andrew lippman mit
Andrew lippman mit Viral entry
Viral entry Viral recombination
Viral recombination Inmunidad
Inmunidad Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Mobilivirus
Mobilivirus Viral arthritis
Viral arthritis Equine viral rhinopneumonitis
Equine viral rhinopneumonitis When blood sample hemolyzed
When blood sample hemolyzed Inklüzyon cisimcikleri
Inklüzyon cisimcikleri Berkehendak
Berkehendak Voltērs
Voltērs Maha khachab
Maha khachab Iman dalam bahasa arab
Iman dalam bahasa arab