VIRAL HAEMORRHAGIC FEVER PREPAREDNESS AND RESPONSE IN EMERGENCIES


























- Slides: 36

VIRAL HAEMORRHAGIC FEVER: PREPAREDNESS AND RESPONSE IN EMERGENCIES DR. ROBERT OBI

OUTLINE Introduction 2 VHF Families Transmission Influence of the environment Vectors of VHFs Risk factors Epidemiology Pathogenesis Incidence in Nigeria Treatment Preparedness and response Conclusion Recommendations 1 3/6/2021

INTRODUCTION 3 Viral hemorrhagic fever (VHF) is a syndrome caused by infection with one of four distinct families of enveloped RNA viruses. It results in increased permeability of the blood vessels, leading to bleeding into the skin (purpura), internal organs mouth and gums, eyes and urine (Bannister, 2010). The viral families implicated in VHFs include: Arenaviridae, Bunyaviridae, Filoviridae and Flaviviridae. These viral families are all classified as biosafety level four (BSL-4) pathogens requiring the highest level of laboratory containment. VHF viruses are restricted in their geographic range by the limitations of their natural host species, which are usually rodents, 1 insects, bats and other mammals (Bannister, 2010) 3/6/2021

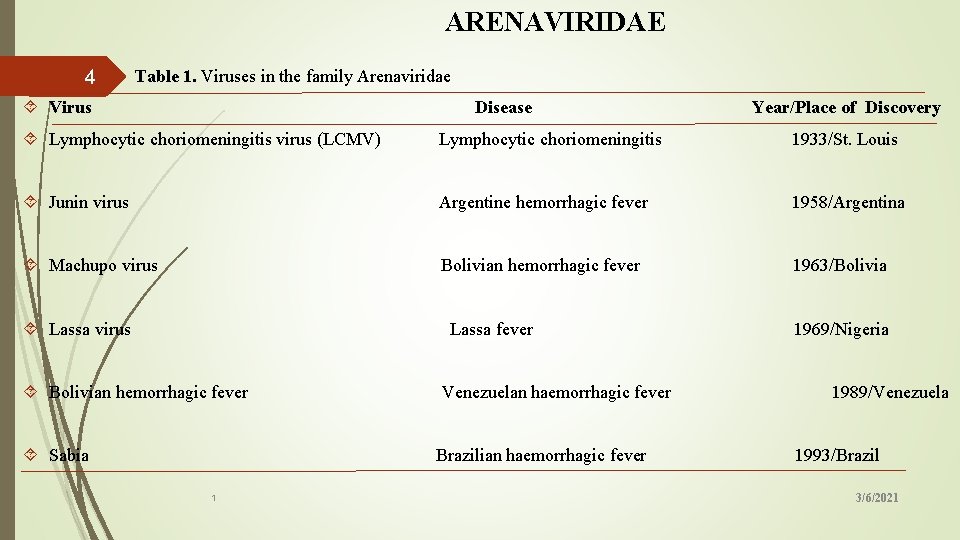
ARENAVIRIDAE Table 1. Viruses in the family Arenaviridae 4 Virus Disease Year/Place of Discovery Lymphocytic choriomeningitis virus (LCMV) Lymphocytic choriomeningitis 1933/St. Louis Junin virus Argentine hemorrhagic fever 1958/Argentina Machupo virus Bolivian hemorrhagic fever 1963/Bolivia Lassa virus Lassa fever 1969/Nigeria Bolivian hemorrhagic fever Venezuelan haemorrhagic fever 1989/Venezuela Sabia Brazilian haemorrhagic fever 1993/Brazil 1 3/6/2021

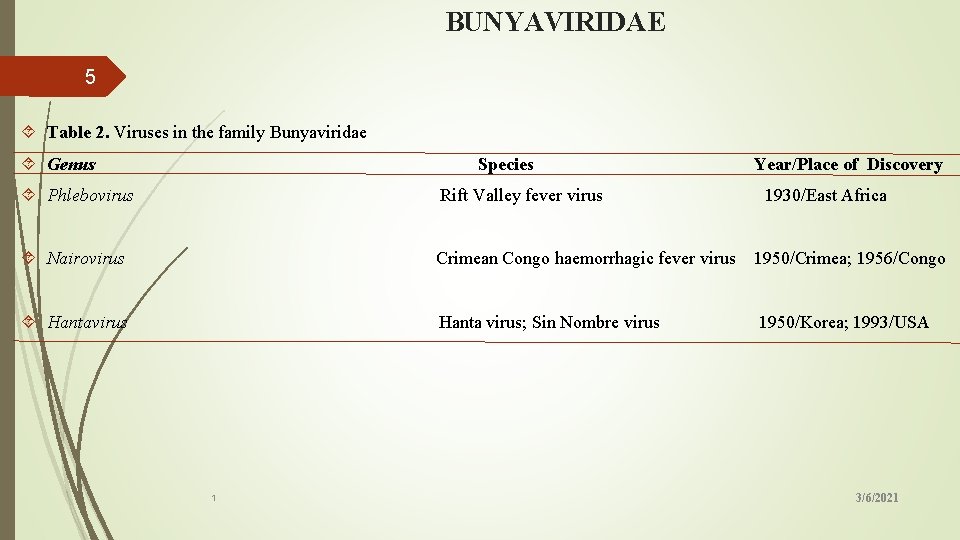
BUNYAVIRIDAE 5 Table 2. Viruses in the family Bunyaviridae Genus Species Phlebovirus Year/Place of Discovery Rift Valley fever virus 1930/East Africa Nairovirus Crimean Congo haemorrhagic fever virus 1950/Crimea; 1956/Congo Hantavirus Hanta virus; Sin Nombre virus 1950/Korea; 1993/USA 1 3/6/2021

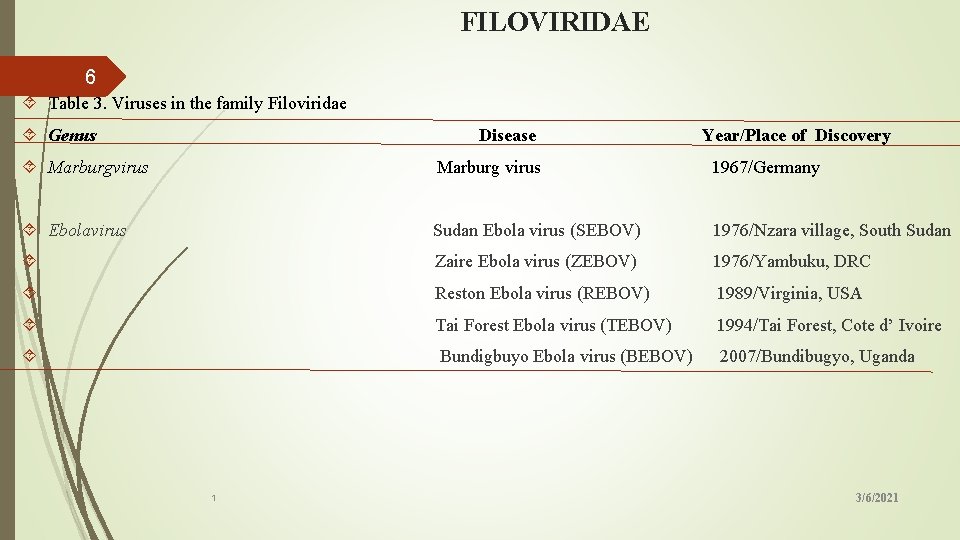
FILOVIRIDAE 6 Table 3. Viruses in the family Filoviridae Genus Disease Year/Place of Discovery Marburgvirus Marburg virus 1967/Germany Ebolavirus Sudan Ebola virus (SEBOV) 1976/Nzara village, South Sudan Zaire Ebola virus (ZEBOV) 1976/Yambuku, DRC Reston Ebola virus (REBOV) 1989/Virginia, USA Tai Forest Ebola virus (TEBOV) 1994/Tai Forest, Cote d’ Ivoire Bundigbuyo Ebola virus (BEBOV) 2007/Bundibugyo, Uganda 1 3/6/2021

FLAVIVIRIDAE 7 Viruses in this family are insect borne While Yellow Fever, Dengue Fever, Japanese encephalitis, West Nile viruses, and Zika virus are mosquito borne especially Aedes and Culex species (Juliano and Lounibos, 2016). Tick-borne Encephalitis (TBE), Kyasanur Forest Disease (KFD) and Alkhurma disease, and Omsk hemorrhagic fever are tick-borne 1 3/6/2021

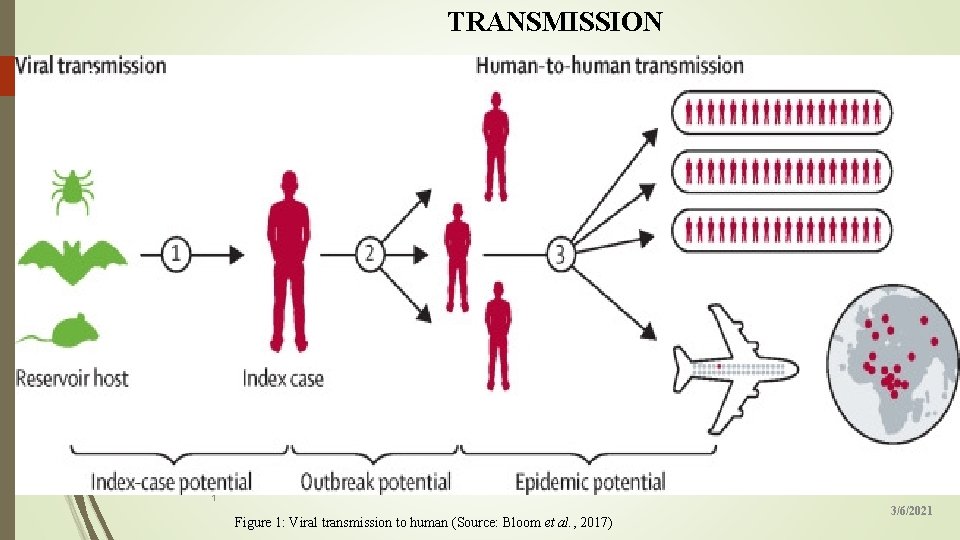
TRANSMISSION 8 1 Figure 1: Viral transmission to human (Source: Bloom et al. , 2017) 3/6/2021

TRANSMISSION CONT’D 9 1 Figure 2. Transmision of Lassa fever (Ilesanmi et al. , 2015) 3/6/2021

INFLUENCE OF THE ENVIRONMENT ON TRANSMISSION 10 Climate change is a major factor for emergence of new infectious agents Spread of new and old infectious agents into new regions and territories (Pica and Bouvier, 2012). Because of global warming, virus-carrying arthropods and ticks may expand into new territories unprepared for their arrival. The same is true for virus-carrying vertebrates such as rodents, bats and birds (Oluwayelu, 2013). Deforestation can result in increased human contact with wild life, leading to exposure to zoonoses (Wolfe et al. , 2016) Delay in collection, treatment and disposal of solid waste result in pollution of soil, water and air, creating breeding grounds for biological vectors and rodents (Onyido et al. , 2009). 1 3/6/2021

INFLUENCE OF THE ENVIRONMENT 11 1 Figure 3. Climatic factors 3/6/2021

INFLUENCE OF THE ENVIRON CONT’D 12 1 Figure 4. Climatic factors 3/6/2021

VECTORS OF VHFs 13 1 Figure 5. Vectors of VHF a) Masotmys natalensis (Lassa fever virus) b) Apodemus agrarius (Hanta fever virus) c) Hypsignathus monstrosus (Ebola 3/6/2021 virus)

VECTORS OF VHFs CONT’D 14 1 Figure 5 d) Hyaloma marginatum maginatum CCHFV e) Aedes aegypti YFV, Zika virus, DFV RFV f) Culex tarsalis WNFV 3/6/2021

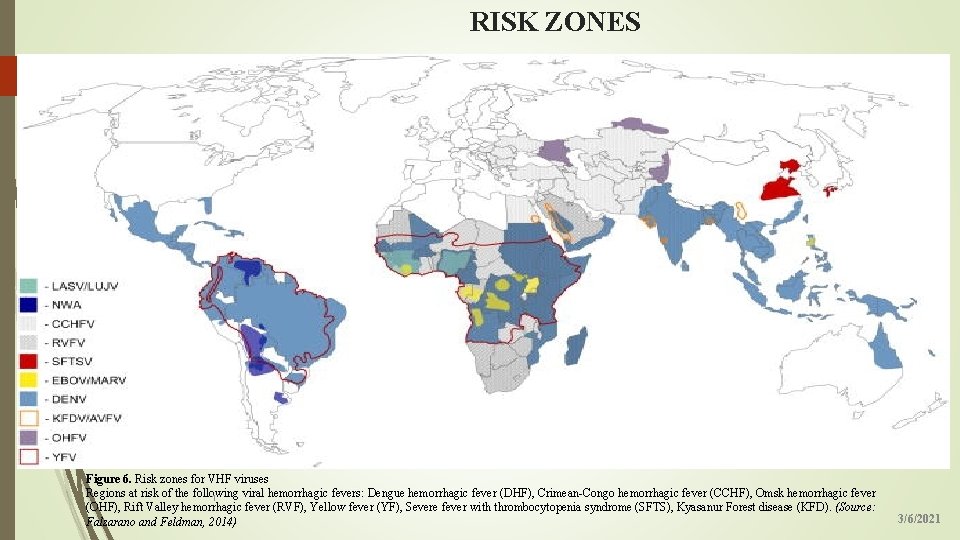
RISK ZONES 15 Figure 6. Risk zones for VHF viruses Regions at risk of the following viral hemorrhagic fevers: Dengue hemorrhagic fever (DHF), Crimean-Congo hemorrhagic fever (CCHF), Omsk hemorrhagic fever 1 (OHF), Rift Valley hemorrhagic fever (RVF), Yellow fever (YF), Severe fever with thrombocytopenia syndrome (SFTS), Kyasanur Forest disease (KFD). (Source: Falzarano and Feldman, 2014) 3/6/2021

EPIDEMIOLOGY 16 v Globally, 2. 5 billion people are at risk of Dengue fever with 40 -80 million annual infection rate leading to thousands of deaths, about 78, 0000 (Hartman et al. , 2010). v Approximately 200, 000 cases of yellow fever occur annually, resulting in about 30, 000 to 60, 000 deaths, with nearly 90% of these occurring in tropical and subtropical areas of South America and Sub Saharan Africa (CDC, 2018). v Lassa fever is endemic in parts of West Africa and is responsible for an estimated 300, 000 – 500, 000 infections annually, with 5, 000 deaths (CDC, 2015). v Ebola virus largest outbreak occurred in several countries in West Africa including Nigeria between 2014 and 2015 1 with 28, 646 reported cases and 11, 323 deaths (Oladejo et al. , 2016). 3/6/2021

PATHOGENESIS AND PATHOLOGY 17 Key targets of VHFs are dendritic cells, mononuclear cells and macrophages of the immune system, and vascular endothelial cells as well as hepatocytes and kidney cells (Ergonul and Holbrook, 2011). Patients with VHF may present with abrupt onset of fever, myalgias, and prostration, followed in severe forms by coagulopathy with a petechial rash or ecchymoses and, sometimes overt bleeding (Wahl-Jensen et al. , 2011). Gastrointestinal symptoms commonly observed include diarrhea, vomiting and abdominal pain. Vascular endothelial damage leads to shock and pulmonary edema with liver injury. The death rate of VHF depends on the cause. For Lassa fever it is around 1 -15% in hospitalized people, whereas it is 50 -90% in outbreaks of Ebola virus disease. 1 3/6/2021

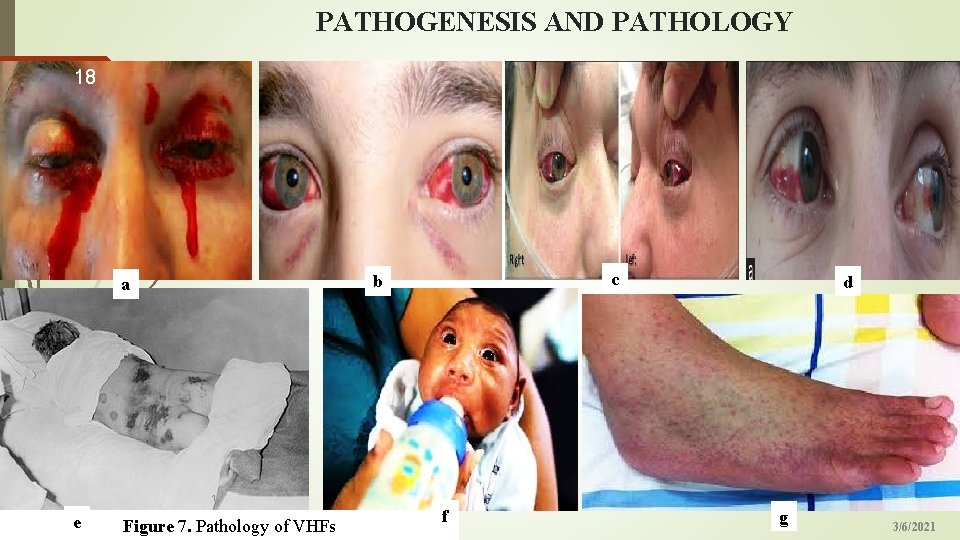
PATHOGENESIS AND PATHOLOGY 18 c b a d 1 e Figure 7. Pathology of VHFs f g 3/6/2021

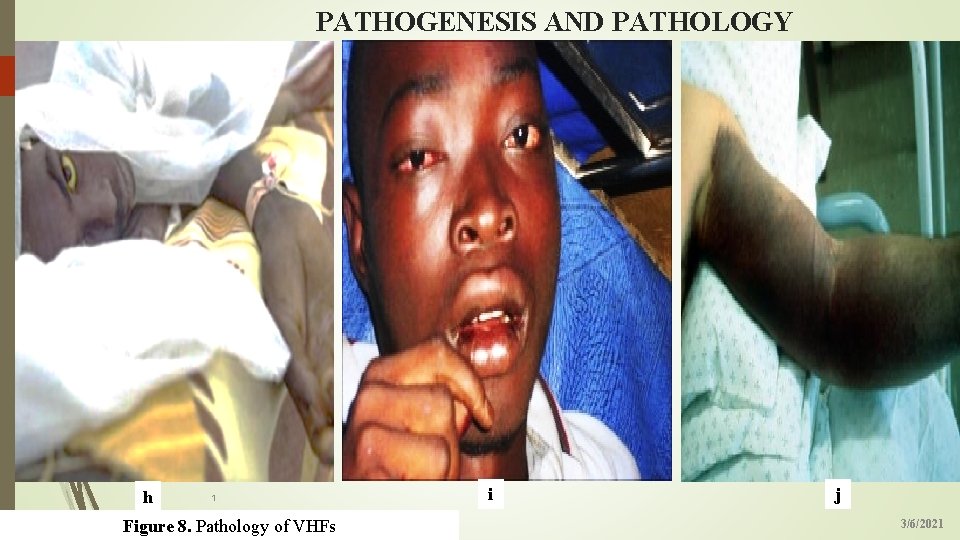
PATHOGENESIS AND PATHOLOGY 19 h 1 Figure 8. Pathology of VHFs i j 3/6/2021

INCIDENCE OF VHFs IN NIGERIA The most prevalent VHFs in Nigeria are Lassa fever, Ebola, Dengue fever and Yellow fever. 20 One thousand six hundred and forty (1, 640) cases of yellow fever were reported in seven states in Nigeria in 2018. They include Kogi, Nasarawa, Anambra, Zamfara, Edo, and Benue states and the Federal Capital Territory, with 43 confirmed cases and nine deaths in Edo (NCDC, 2018). Nigeria reported the largest number of Lassa fever cases in 2018, with 600 confirmed cases and 170 deaths (NCDC, 2018). The states with the highest annual prevalence rate of Lassa fever outbreaks include Nigeria include Edo, Ondo, Bauchi, Nasarawa, Ebonyi, Plateau, Taraba, FCT, Adamawa, Gombe, Kaduna, Kwara, Benue, Rivers, Kogi, Enugu, Imo, Delta, Oyo, and Kebbi. Ebola virus disease outbreak occurred in Nigeria for the first time in 2014 with 19 confirmed cases and 8 deaths in Lagos and River States. Dengue haemorhagic fever is highly prevalent in Nigeria and have been reported in Cross River, Akwa Ibom, Benue, Rivers, Taraba, 1 Anambra, Enugu, Abia, Delta and Nasarawa States (Ayukekbong, 2015). 3/6/2021

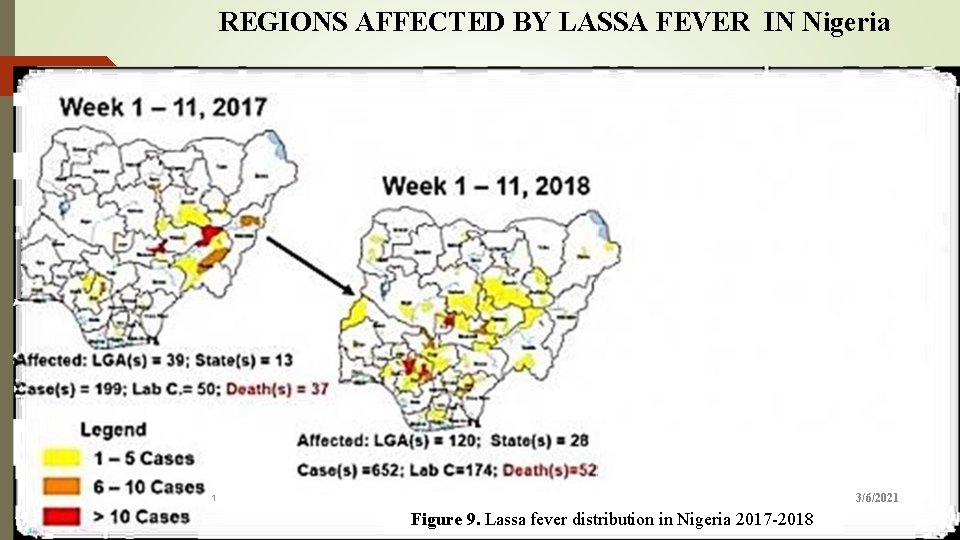
REGIONS AFFECTED BY LASSA FEVER IN Nigeria 21 2. 6 : Map of Nigeria showing areas affected by Lassa fever, week 1 -11, 2017 & 2018. Figure Source: (Mc. Cormick et al; 2018) 3/6/2021 1 Figure 9. Lassa fever distribution in Nigeria 2017 -2018

TREATMENT 22 There are no antiviral drugs for treatment of VHFs, although Ribavirin, a nucleoside analog, has some in vitro and in vivo activity against Arenaviridae and Bunyaviridae but has no effect on Filoviridae or Flaviviridae (Chakrabarti, D. and Banik, S. 2012). Fluid resuscitation and supportive care the mainstays of emergency department therapy. Intravenous crystalloids, oxygen, and cardiac monitoring are the most appropriate initial steps in the treatment of patients in whom viral hemorrhagic fever (VHF) is suggested. Other measures include the following: Administering blood and blood products as clinically indicated Avoiding intramuscular injections and the use of aspirin or other anticoagulants Minimizing invasive procedures because of the risk associated with viral transmission from sharp objects Minimizing aerosol-generating procedures such as bilevel positive airway pressure (bi. PAP), intubation, bronchoscopy and sputum induction (Rasca et al. , 2016). 1 3/6/2021

PREPAREDNESS AND RESPONSE IN EMERGENCIES 23 VHF preparedness and response in emergencies is the process of anticipating, preventing, preparing for, detecting, responding, and controlling VHFs in order that the health and economic impacts are minimized (Isere et al. , 2015). A VHF preparedness plan is a written document giving decision-makers and other key players orientation on a list of activities to be implemented in order to respond to VHFs (FMo. H, 2009). A National VHF preparedness plan identifies and describes systems, activities, resources and timelines at National, State and Local Government Area levels (NCDC, 2016). Key operational areas of the preparedness are coordination, epidemiology and surveillance, capacity building for case management, strengthening laboratory capacity, logistics, communication and social mobilization, point of entry and research (Freedman et al. , 2014). 3/6/2021

PREPAREDNESS CONT’D 24 COORDINATION: Strong central coordination is essential to the control of disease outbreaks. The coordinating team provides the strategic directions for the responses which are implemented by the technical subgroups and also liaises with the media and the political authorities The coordination of VHFs preparedness activities at the National level is the responsibility of the NCDC. This is done through the establishment of functional EOCs. At the State level, this responsibility lies with the Director of Public Health or delegated to the State Epidemiologist (NCDC, 2016). NCDC recommends that all States set-up and maintain EOCs. State Governments and LGAs have the responsibility to initiate and institute actions on issues of disease prevention, control and response. 3/6/2021

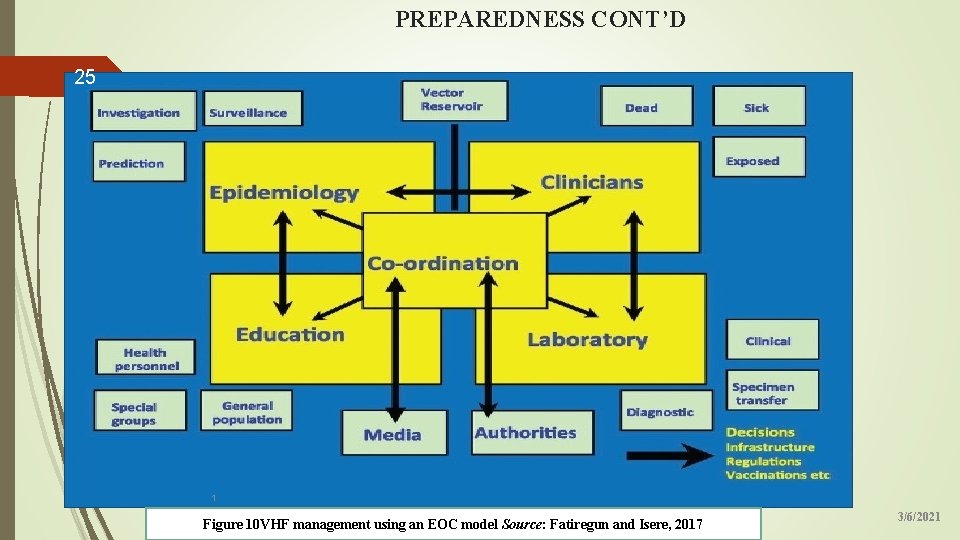
PREPAREDNESS CONT’D 25 1 Figure 10 VHF management using an EOC model Source: Fatiregun and Isere, 2017 3/6/2021

PREPAREDNESS CONT’D 26 EPIDEMIOLOGY AND SURVEILLANCE: The VHF surveillance system is designed to detect cases of VHF in a timely manner to enable prompt verification, investigation and response thereby minimizing spread of these viruses in the community (Frieden et al. , 2015). CAPACITY BUILDING FOR CASE MANAGEMENT: This involves strengthening or building capacity for prompt management of suspected or confirmed VHF cases (Fatiregun and Isere, 2017). STRENGTHENING LABORATORY CAPACITY: This involves strengthening the laboratory capacity for optimal contribution to countrywide VHF surveillance COMMUNICATION 1 AND SOCIAL MOBILIZATION: Develop and ensure availability of capacity for public awareness, risk communication, and community engagement for VHF prevention (NCDC, 2017). 3/6/2021

PREPAREDNESS CONT’D 27 POINT OF ENTERY (POE): Preparedness activities within Port Health Services and at Po. E aim to immediately detect, verify/investigate and effectively manage potential imported cases of VHF into Nigeria, and to limit further transmission and spread within the country. LOGISTICS MANAGEMENT: Ensure availability and access to funds, logistics, supplies and appropriate equipment for the immediate and initial response to surveillance of VHF in Nigeria (NCDC, 2017). TRAINING AND RESEARCH: This involves building national capacities for preparedness, prevention and control for VHF through training. It entails delivering focused trainings targeting different categories of health care workers with responsibilities for preparedness, prevention and control of VHF. 1 3/6/2021

RESPONSE 28 The VHF response system is designed to detect cases of VHF in a timely manner to enable prompt verification, investigation and response thereby minimizing spread of these viruses in the community. At the national level, it involves Strengthening case management and laboratory diagnosis Providing technical support to States in terms of strategy and technical support to strengthen VHF outbreak response. NCDC will also provide supportive supervision in all aspects of VHF response: administrative, logistics and finance management for operations. Coordinate and support the activation of previously identified VHF isolation centres and promote access to free treatment by patients with VHF. 1 Coordinate the organization of support for health-care workers and deployed staff that are at higher risk of being infected. 3/6/2021

RESPONSE CONT’D 29 States should activate the EOC/RRT. The RRT will coordinate VHF response activities at the respective levels of operations. RRTs are in charge of supporting local response operations and should be adaptable to the evolving situation. RRT should comprise of different professionals from all relevant sectors. Persons with prior experience on VHF response should be included if available. States and LGAs should work in line with national guidelines and be the first to initiate and institute actions on issues of 1 disease prevention, control and response. 3/6/2021

RESPONSE CONT’D 30 NOTIFICATION OF VHF OCCURRENCE: The response to a VHF case requires efficient and coordinated communication at all levels (LGA, States and National). The Infectious Disease Surveillance Response (IDSR) flow of information shall be used. Health center Physician Reporting LGA Disease Surveillance Notification Officer (DNSO) Reporting State Epidemiologist and NCDC National Reporting WHO 1 (B) ACTIVE CASE SEARCH AND CONTACT TRACING 3/6/2021

RESPONSE CONT’D CASE MANAGEMENT: This is undertaken to strengthen or build capacity for prompt management of suspected 31 or confirmed VHF cases at national, State and LGA levels. Treatment: Any suspected VHF case should be admitted immediately by the managing clinician into an isolation room/ward, in a hospital with the capacity to treat severely ill patients and laboratory capacity for specimen collection. Blood specimen should be collected immediately by the managing clinician from all VHF suspected cases and sent to the laboratory for confirmation. Ribavirin is the drug of choice for the treatment of Lassa fever. For treatment to be effective, it should be initiated early within the first week of onset of illness. 1 3/6/2021

RESPONSE CONT’D 32 SAFE BURIAL PRACTICES: There is a major risk of transmission when a patient dies of VHFs, as the dead body remains contagious for several days after death. The burial must take place as early as possible after preparation of the remains at the hospital. INFECTION PREVENTION AND CONTROL (IPC): Infection prevention control committee shall appoint and oversee IPC monitoring and compliance officers in charge of each unit of the treatment center. The monitoring and compliance officers shall ensure strict adherence to the IPC policy, guidelines and SOPs as it relates to the services provided by each unit of care 1 3/6/2021

RESPONSE CONT’D 33 LABORATORY SERVICE: This is initiated to strengthen the laboratory capacity for optimal contribution to countrywide VHF outbreak response. COMMUNICATION AND SOCIAL MOBILIZATION: Ensures prompt communication to the public on the outbreak, importance of early identification and reporting to designated health authorities/facilities for help and treatment, as well as scale up messaging for community and all stakeholders’ participation in detection, prevention and control. POINT OF ENTERY (POE): Activities within Port Health Services and at Po. E aim to immediately detect, verify/investigate and effectively manage potential imported cases of VHF into Nigeria, and to limit further transmission and spread within the country 1 3/6/2021

CONCLUSION 34 v Preparing to respond to VHF and other public health events is an important part of health-care delivery services. v However, to be able to implement a coordinated disease outbreak response that will be timely and effective to prevent unnecessary deaths and disabilities, especially in resource-limited settings during disease outbreaks, v an emergency operation center (EOC) model can be adopted by public health expert as it has proven to be an efficient preparedness framework for the control of disease outbreaks such as Ebola Virus Disease and other public health events in Nigeria and several 1 African countries. 3/6/2021

RECOMMEDATIONS 35 The LGA system in Nigeria is a very important part of health care, it should not be allowed to go into extinction. The three tiers of government in Nigeria should ensure total enforcement of the National environmental exercise last Saturday of every month. This will guarantee clean environments and reduce the breeding ground of vectors of VHFs. The surveillance at the ports should be strengthened to ensure prompt detection of VHF cases. There should be constant training and re-training of health-care workers to prepare them for any emergency and unexpected outbreak of VHFs 1 3/6/2021

36 1 3/6/2021
 Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever Preparedness mitigation response recovery
Preparedness mitigation response recovery Section 24-1 viral structure and replication
Section 24-1 viral structure and replication Chapter 36 emergency preparedness and protective practices
Chapter 36 emergency preparedness and protective practices Chapter 36 emergency preparedness and protective practices
Chapter 36 emergency preparedness and protective practices Disaster management
Disaster management Natural and forced response
Natural and forced response Natural and forced response
Natural and forced response Primary immune response and secondary immune response
Primary immune response and secondary immune response Lesson 6: cardiac emergencies and using an aed
Lesson 6: cardiac emergencies and using an aed Endocrine and hematologic emergencies
Endocrine and hematologic emergencies Emt chapter 18 gastrointestinal and urologic emergencies
Emt chapter 18 gastrointestinal and urologic emergencies Chapter 28 first aid and emergencies
Chapter 28 first aid and emergencies Viral induced wheeze vs asthma
Viral induced wheeze vs asthma Replicação viral ciclo lítico e lisogênico
Replicação viral ciclo lítico e lisogênico Pantropizm
Pantropizm Method of cultivation of virus
Method of cultivation of virus Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Egg inoculation diagram
Egg inoculation diagram Spasmodic croup
Spasmodic croup Dea anggraini viral
Dea anggraini viral Morfologia viral
Morfologia viral When blood sample hemolyzed
When blood sample hemolyzed Bacilos gram positivos meningitis
Bacilos gram positivos meningitis Ciclo viral
Ciclo viral Capsid capsomere
Capsid capsomere Viral
Viral Viral recombination
Viral recombination Vaccins à vecteur viral
Vaccins à vecteur viral Viral receptors
Viral receptors Streptococcus
Streptococcus Equine viral rhinopneumonitis
Equine viral rhinopneumonitis Viral dna
Viral dna Viral gastroenteritis
Viral gastroenteritis Viral entry
Viral entry The dynamics of viral marketing
The dynamics of viral marketing Hgado
Hgado