Unit Processes Lecture1 Types of Organic Reactions Types





- Slides: 21

Unit Processes Lecture-1 Types of Organic Reactions

Types of Organic Reactions • Addition Reaction • Elimination Reaction • Substitution Reaction • Condensation Reaction • Esterification Reaction • Hydrolysis Reaction • Oxidation Reaction • Reduction Reaction

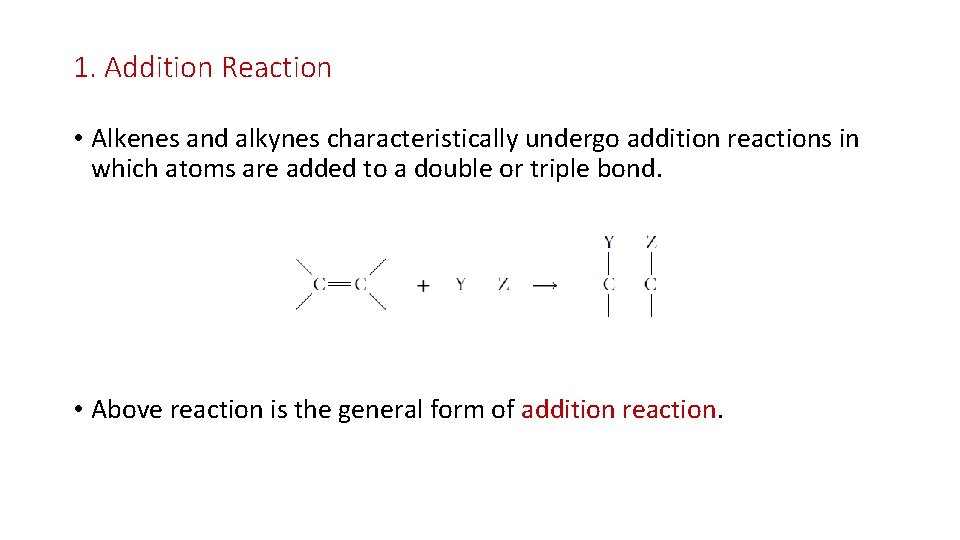
1. Addition Reaction • Alkenes and alkynes characteristically undergo addition reactions in which atoms are added to a double or triple bond. • Above reaction is the general form of addition reaction.

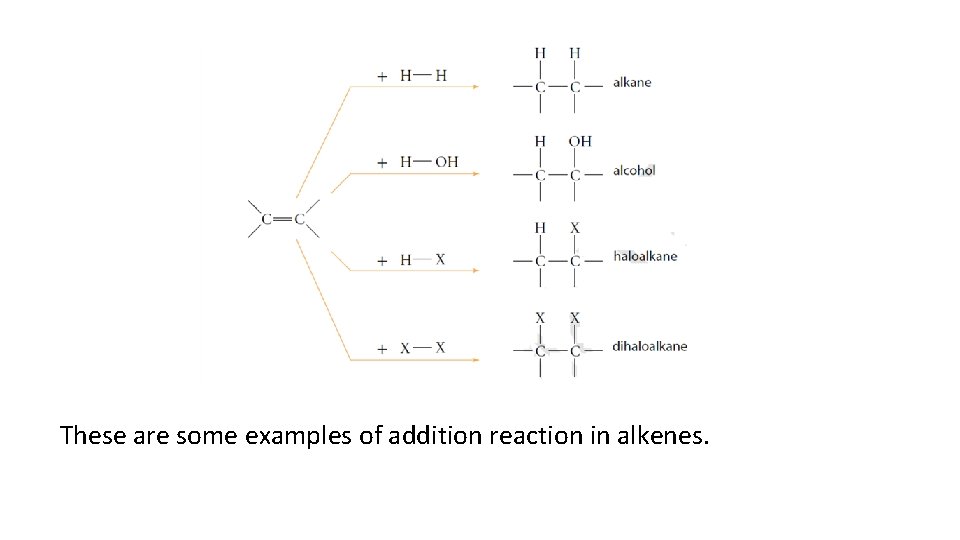
These are some examples of addition reaction in alkenes.

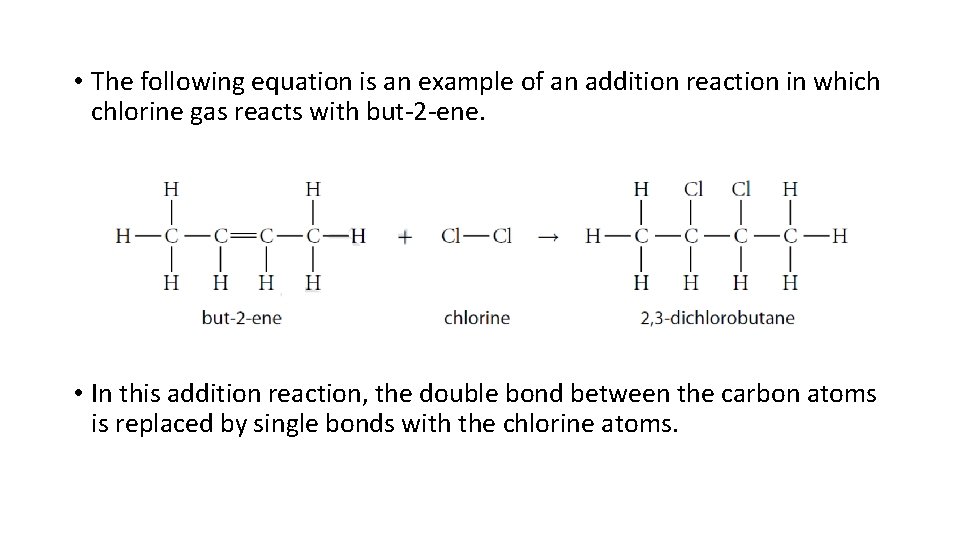
• The following equation is an example of an addition reaction in which chlorine gas reacts with but-2 -ene. • In this addition reaction, the double bond between the carbon atoms is replaced by single bonds with the chlorine atoms.

Addition of Bromine • Chemists use the addition reaction of bromine with an alkene as a colour test for the presence of double or triple bond. • The loss of brownish colour indicates that the compound contains multiple bonds.

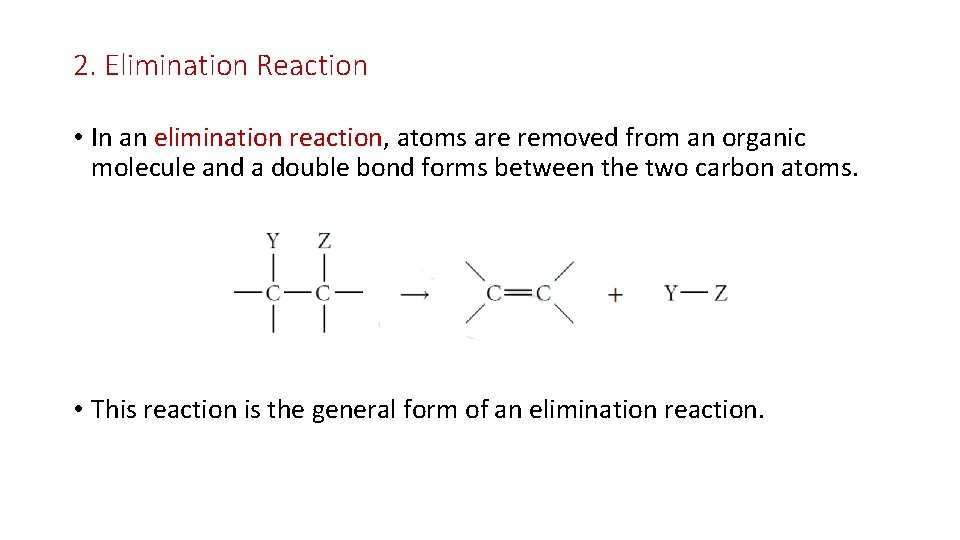
2. Elimination Reaction • In an elimination reaction, atoms are removed from an organic molecule and a double bond forms between the two carbon atoms. • This reaction is the general form of an elimination reaction.

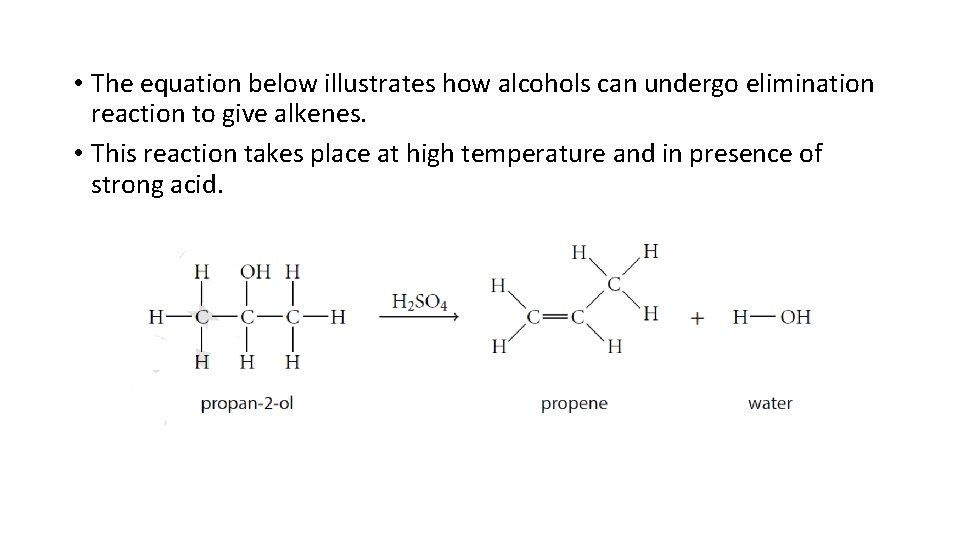
• The equation below illustrates how alcohols can undergo elimination reaction to give alkenes. • This reaction takes place at high temperature and in presence of strong acid.

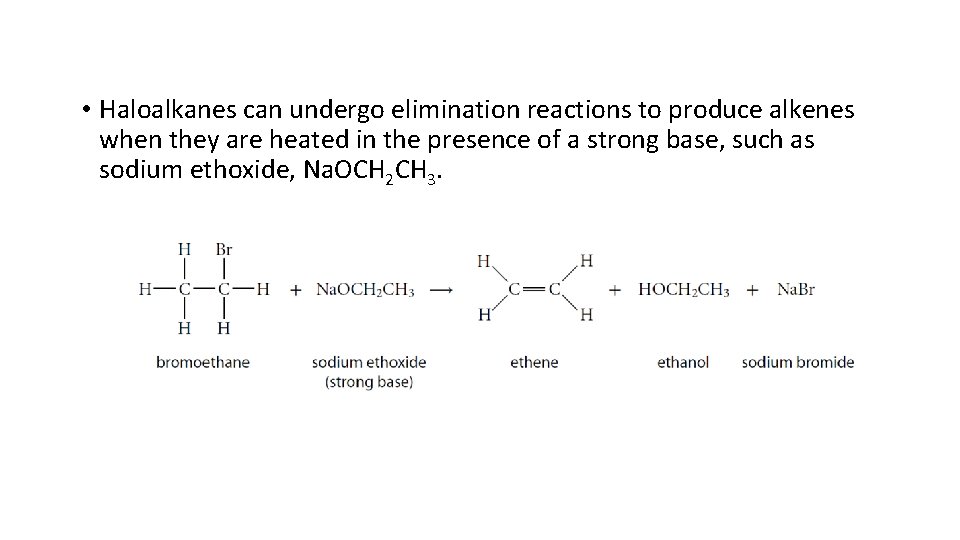
• Haloalkanes can undergo elimination reactions to produce alkenes when they are heated in the presence of a strong base, such as sodium ethoxide, Na. OCH 2 CH 3.

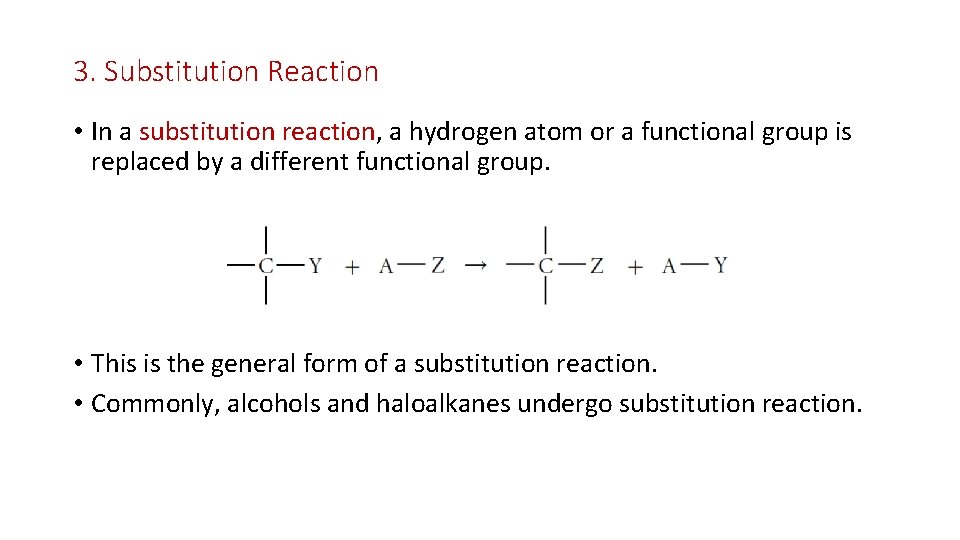
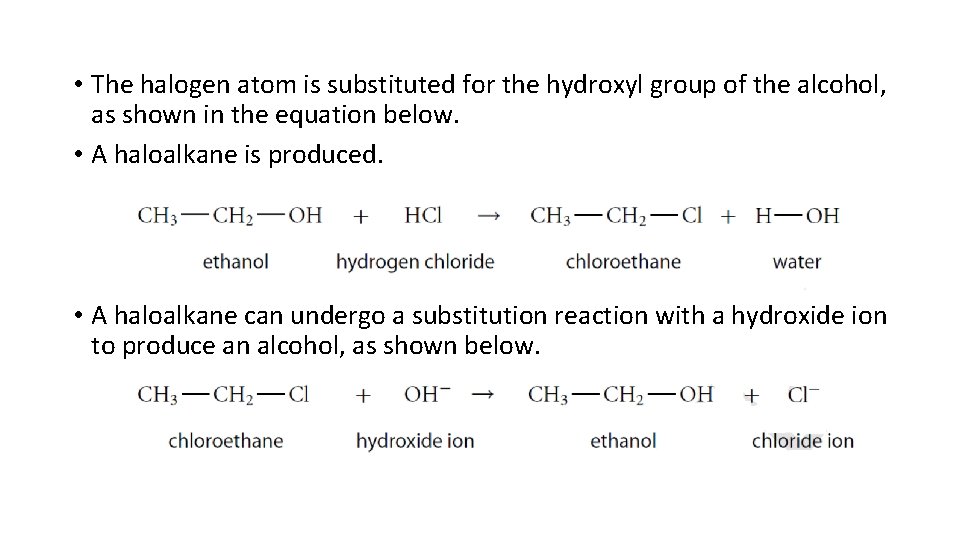
3. Substitution Reaction • In a substitution reaction, a hydrogen atom or a functional group is replaced by a different functional group. • This is the general form of a substitution reaction. • Commonly, alcohols and haloalkanes undergo substitution reaction.

• The halogen atom is substituted for the hydroxyl group of the alcohol, as shown in the equation below. • A haloalkane is produced. • A haloalkane can undergo a substitution reaction with a hydroxide ion to produce an alcohol, as shown below.

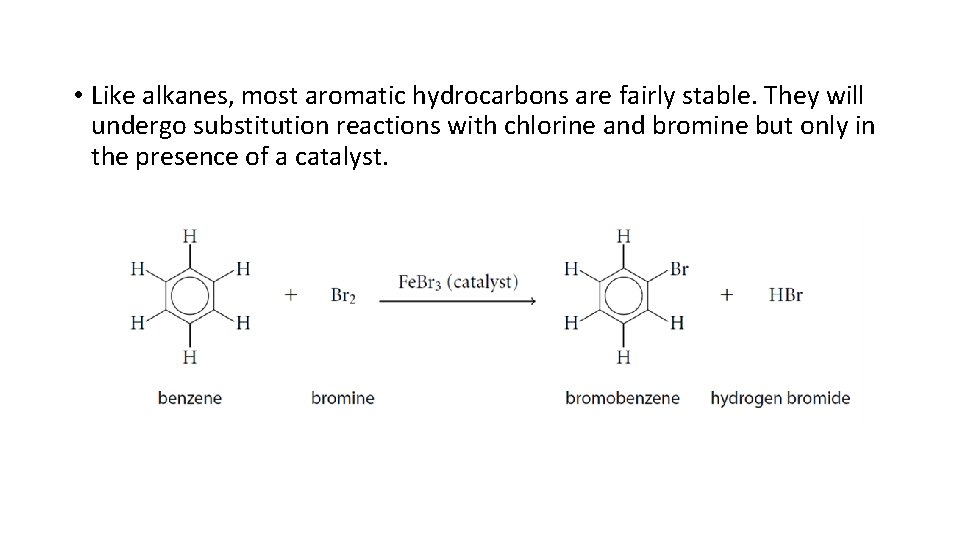
• Like alkanes, most aromatic hydrocarbons are fairly stable. They will undergo substitution reactions with chlorine and bromine but only in the presence of a catalyst.

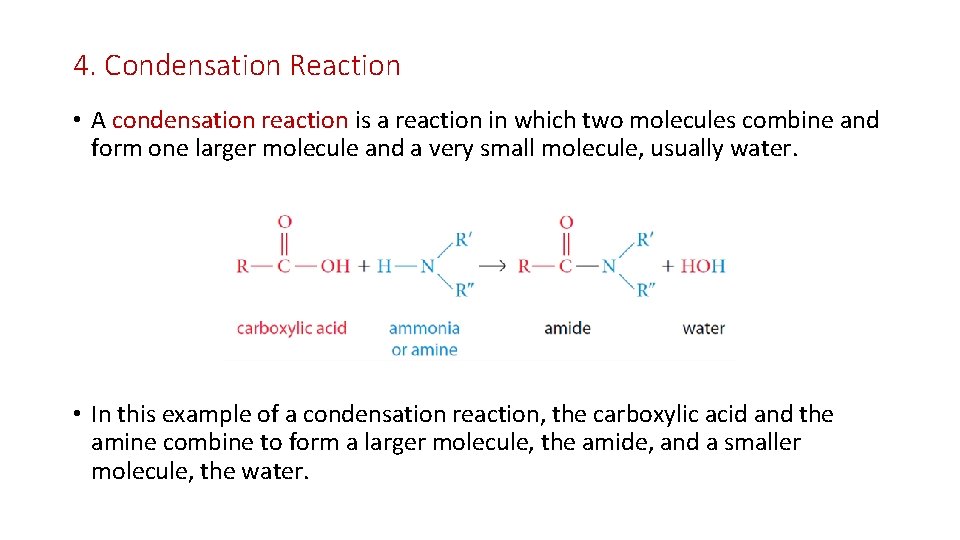
4. Condensation Reaction • A condensation reaction is a reaction in which two molecules combine and form one larger molecule and a very small molecule, usually water. • In this example of a condensation reaction, the carboxylic acid and the amine combine to form a larger molecule, the amide, and a smaller molecule, the water.

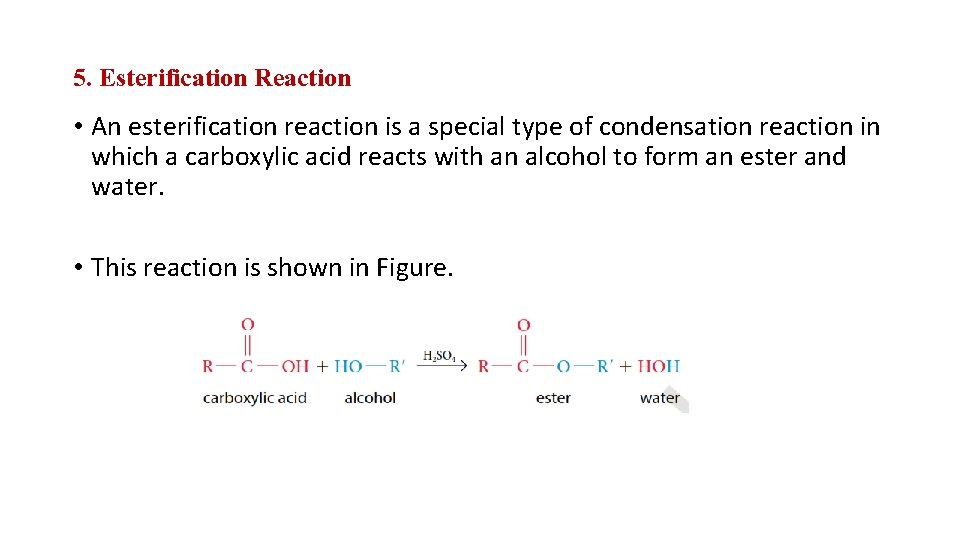
5. Esterification Reaction • An esterification reaction is a special type of condensation reaction in which a carboxylic acid reacts with an alcohol to form an ester and water. • This reaction is shown in Figure.

• The chemical formula for sulphuric acid over the arrow indicates that the reaction is catalysed by a strong acid. • Esterification reactions can be used to produce useful consumer products, such as acetyl salicylic acid, commonly sold as Aspirin. • Many of the flavours and aromas of fruits and spices are due to the presence of esters. • Through esterification reactions, chemists have learned to duplicate natural esters. • Synthesized esters are used to give artificial flavour to juices, candy, and many foods.

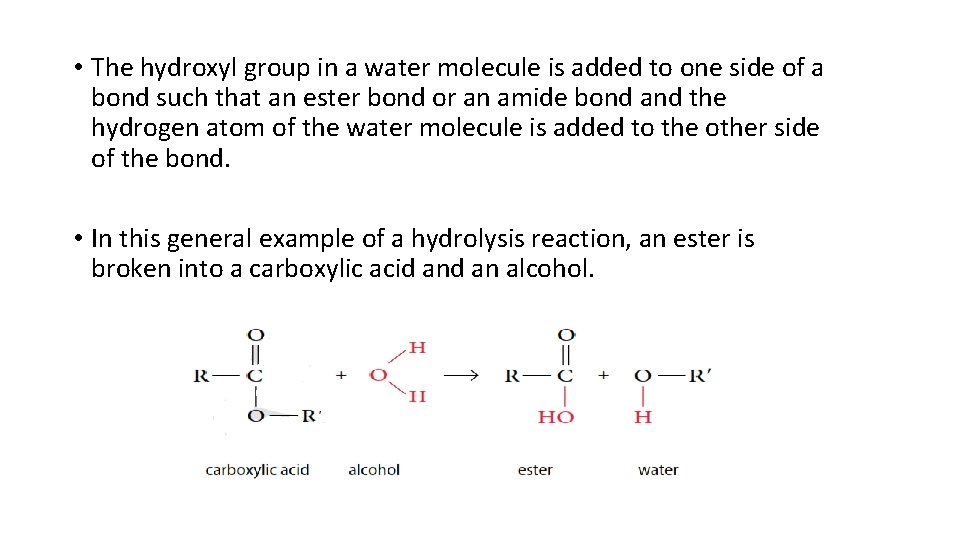
6. Hydrolysis Reaction • The term hydrolysis means to “break apart using water. ” • The compounds that are formed by condensation reactions can be broken down by hydrolysis reactions. • The hydrolysis reaction are catalysed by an acid. • The hydroxyl group in a water molecule is added to one side of a bond.

• The hydroxyl group in a water molecule is added to one side of a bond such that an ester bond or an amide bond and the hydrogen atom of the water molecule is added to the other side of the bond. • In this general example of a hydrolysis reaction, an ester is broken into a carboxylic acid an alcohol.

APPLICATIONS • Typical application is in oil and fats industry during soap manufacture. • Here hydrolysis of fats are carried out to obtain fatty acid and glycerol followed by addition of sodium hydroxide to form soap. • Some of the major product using hydrogen is ethylene from acetylene, methanol, propanol, butanol, production of alcohol from olefins.

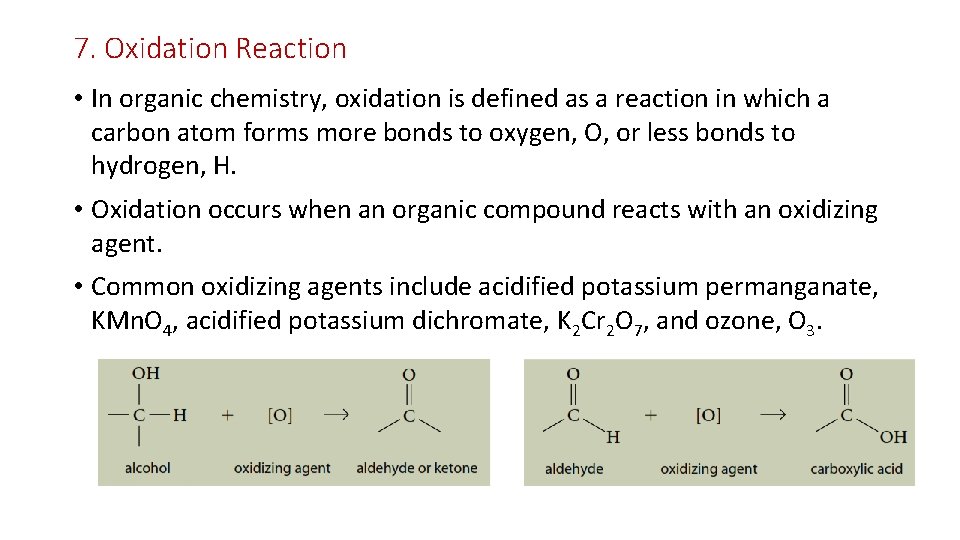
7. Oxidation Reaction • In organic chemistry, oxidation is defined as a reaction in which a carbon atom forms more bonds to oxygen, O, or less bonds to hydrogen, H. • Oxidation occurs when an organic compound reacts with an oxidizing agent. • Common oxidizing agents include acidified potassium permanganate, KMn. O 4, acidified potassium dichromate, K 2 Cr 2 O 7, and ozone, O 3.

8. Reduction Reaction • In organic chemistry, reduction is defined as a reaction in which a carbon atom forms fewer bonds to oxygen, O, or more bonds to hydrogen, H. • C=O bond or C=C double bond is reduced to a single bond by reduction. • Aldehydes, ketones, and carboxylic acids can be reduced to become alcohols. • Alkenes and alkynes can be reduced by the addition of H to become alkanes.

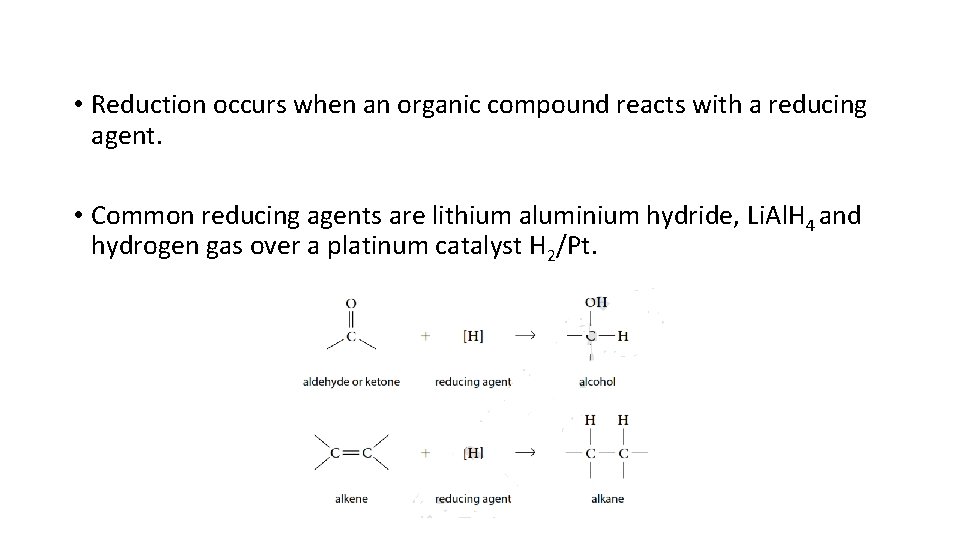
• Reduction occurs when an organic compound reacts with a reducing agent. • Common reducing agents are lithium aluminium hydride, Li. Al. H 4 and hydrogen gas over a platinum catalyst H 2/Pt.