Types of Chemical Reactions Types of Reactions There







- Slides: 17

Types of Chemical Reactions

Types of Reactions • There are five types of chemical reactions we will talk about: 1. 2. 3. 4. 5. • Synthesis reactions Decomposition reactions Single displacement reactions Double displacement reactions Combustion reactions You need to be able to identify the type of reaction and predict the product(s) for some

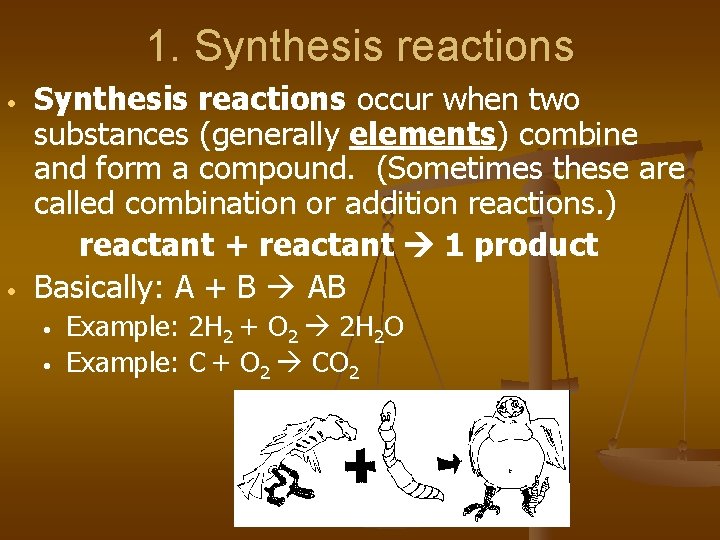
1. Synthesis reactions • • Synthesis reactions occur when two substances (generally elements) combine and form a compound. (Sometimes these are called combination or addition reactions. ) reactant + reactant 1 product Basically: A + B AB • • Example: 2 H 2 + O 2 2 H 2 O Example: C + O 2 CO 2

Synthesis Reactions • Here is another example of a synthesis reaction

Practice • • • Predict the products. Write and balance the following synthesis reaction equations. Sodium metal reacts with chlorine gas Na(s) + Cl 2(g) Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g)

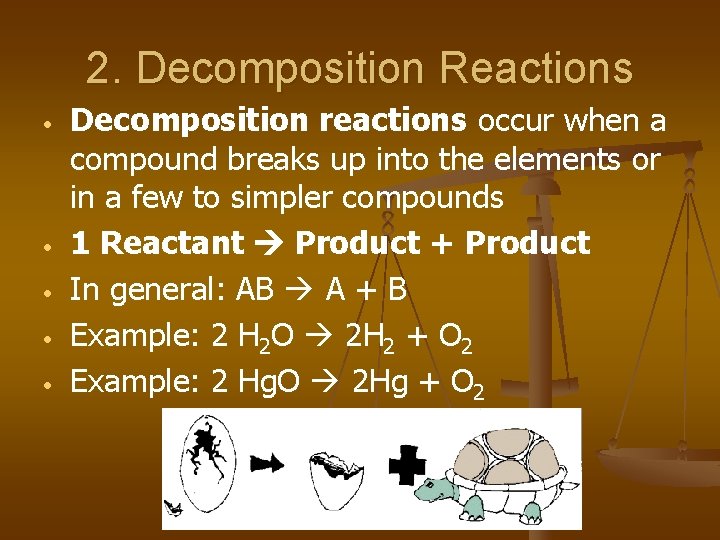
2. Decomposition Reactions • • • Decomposition reactions occur when a compound breaks up into the elements or in a few to simpler compounds 1 Reactant Product + Product In general: AB A + B Example: 2 H 2 O 2 H 2 + O 2 Example: 2 Hg. O 2 Hg + O 2

Decomposition Reactions • Another view of a decomposition reaction:

Practice • • • Predict the products. Then, write and balance the following decomposition reaction equations: Solid Lead (IV) oxide decomposes Pb. O 2(s) Aluminum nitride decomposes Al. N(s)

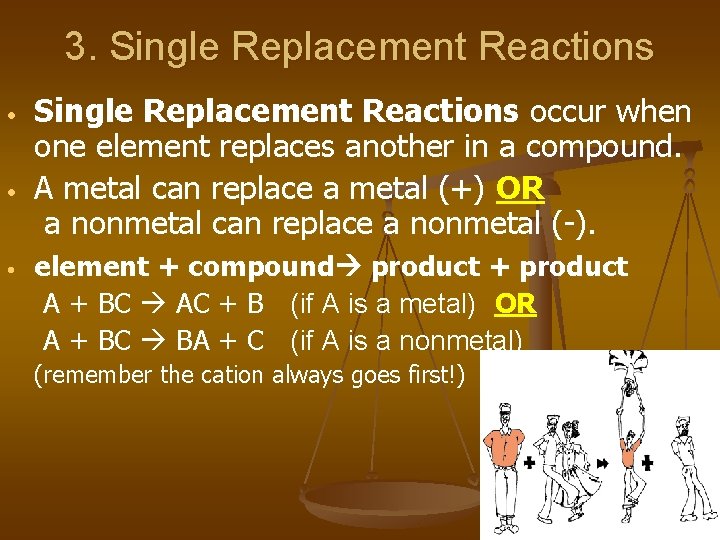
3. Single Replacement Reactions • • • Single Replacement Reactions occur when one element replaces another in a compound. A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). element + compound product + product A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) (remember the cation always goes first!)

Single Replacement Reactions • Another view:

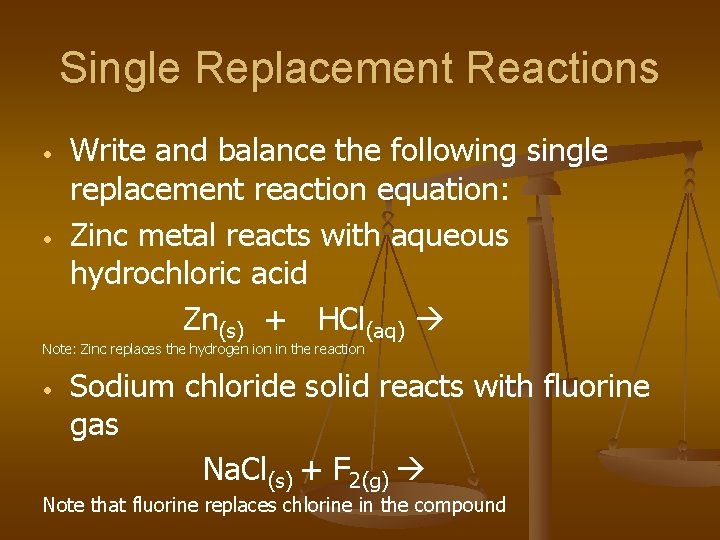
Single Replacement Reactions • • Write and balance the following single replacement reaction equation: Zinc metal reacts with aqueous hydrochloric acid Zn(s) + HCl(aq) Note: Zinc replaces the hydrogen ion in the reaction • Sodium chloride solid reacts with fluorine gas Na. Cl(s) + F 2(g) Note that fluorine replaces chlorine in the compound

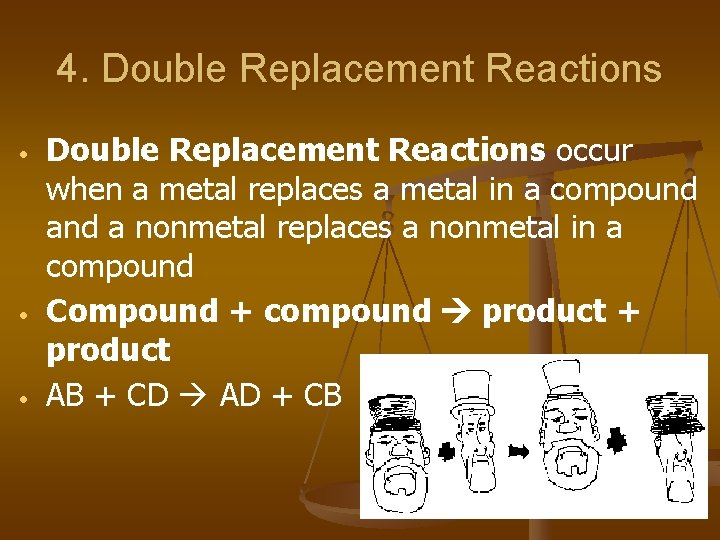
4. Double Replacement Reactions • • • Double Replacement Reactions occur when a metal replaces a metal in a compound a nonmetal replaces a nonmetal in a compound Compound + compound product + product AB + CD AD + CB

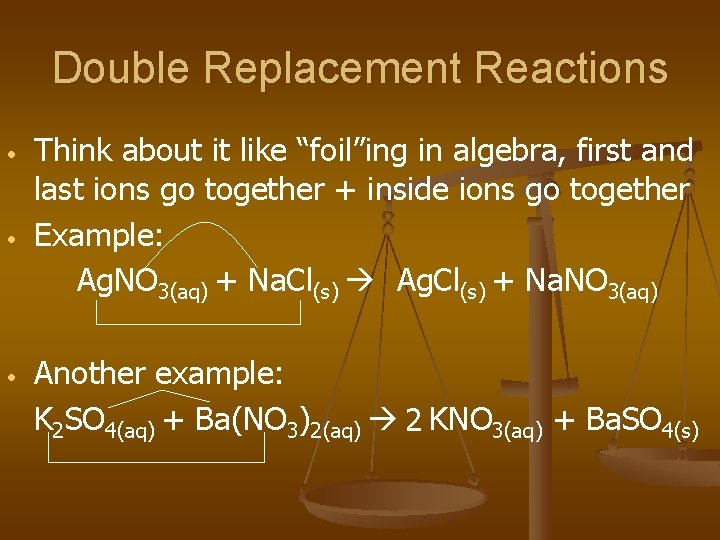
Double Replacement Reactions • • • Think about it like “foil”ing in algebra, first and last ions go together + inside ions go together Example: Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) Another example: K 2 SO 4(aq) + Ba(NO 3)2(aq) 2 KNO 3(aq) + Ba. SO 4(s)

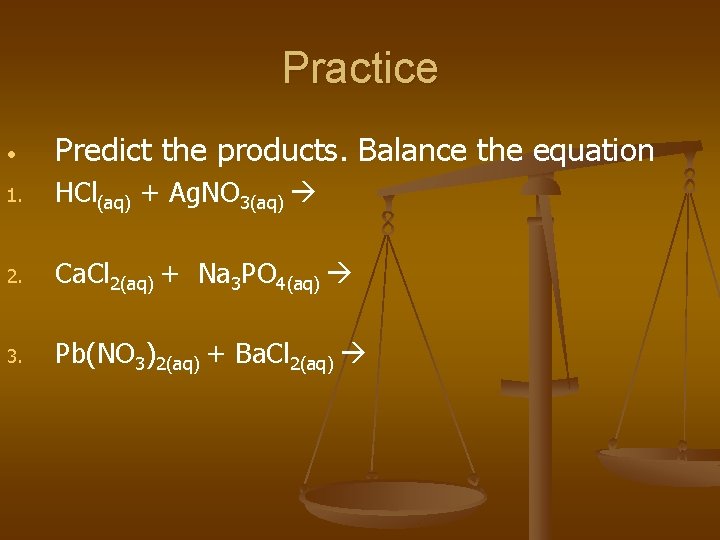
Practice • Predict the products. Balance the equation 1. HCl(aq) + Ag. NO 3(aq) 2. Ca. Cl 2(aq) + Na 3 PO 4(aq) 3. Pb(NO 3)2(aq) + Ba. Cl 2(aq)

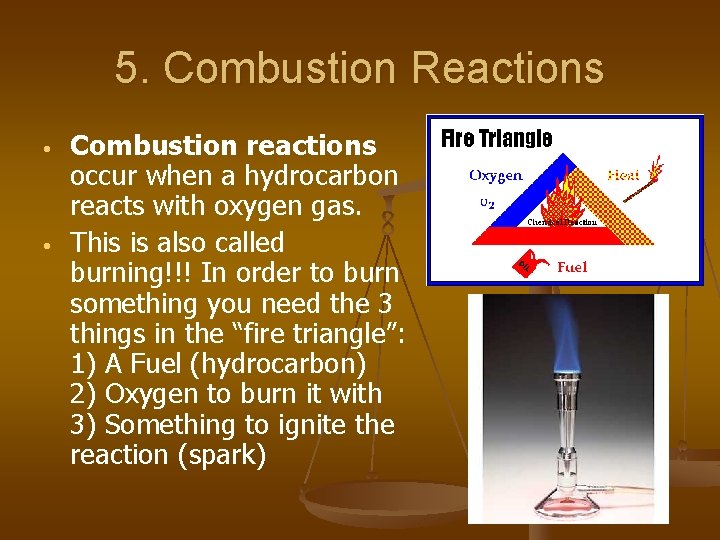
5. Combustion Reactions • • Combustion reactions occur when a hydrocarbon reacts with oxygen gas. This is also called burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel (hydrocarbon) 2) Oxygen to burn it with 3) Something to ignite the reaction (spark)

Combustion Reactions • • • In general: Cx. Hy + O 2 CO 2 + H 2 O Products in combustion are ALWAYS carbon dioxide and water. (although incomplete Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C 8 H 18)

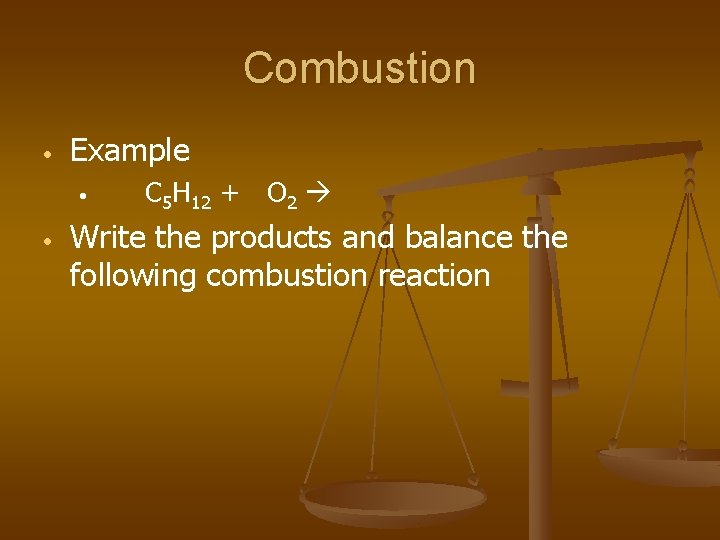
Combustion • Example • • C 5 H 12 + O 2 Write the products and balance the following combustion reaction
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Redox rules
Redox rules How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry Types of reaction
Types of reaction 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What are five chemical changes
What are five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What is the type of reaction
What is the type of reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Fatoumata dembele chef
Fatoumata dembele chef