UNIT 4 REVIEW Physical properties of water Pure


















- Slides: 44

UNIT 4 REVIEW

Physical properties of water Pure water is colourless, odourless, and tasteless. Whether or not an object sinks or floats relates to its density compared to water. – Pure water density = 1. 0 g / m. L

Physical properties of water Water is unique in the fact that its density decreases when it becomes a solid, this is what causes ice to float. This in turn acts a blanket which prevents the water underneath from freezing and killing all life.

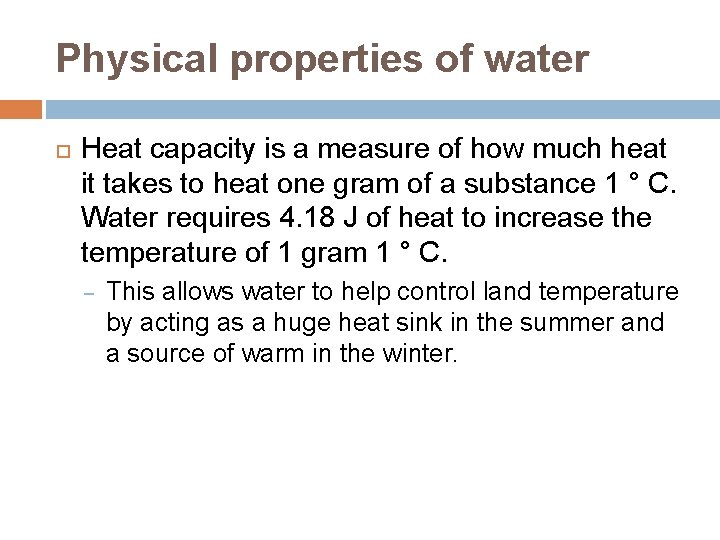
Physical properties of water Heat capacity is a measure of how much heat it takes to heat one gram of a substance 1 ° C. Water requires 4. 18 J of heat to increase the temperature of 1 gram 1 ° C. – This allows water to help control land temperature by acting as a huge heat sink in the summer and a source of warm in the winter.

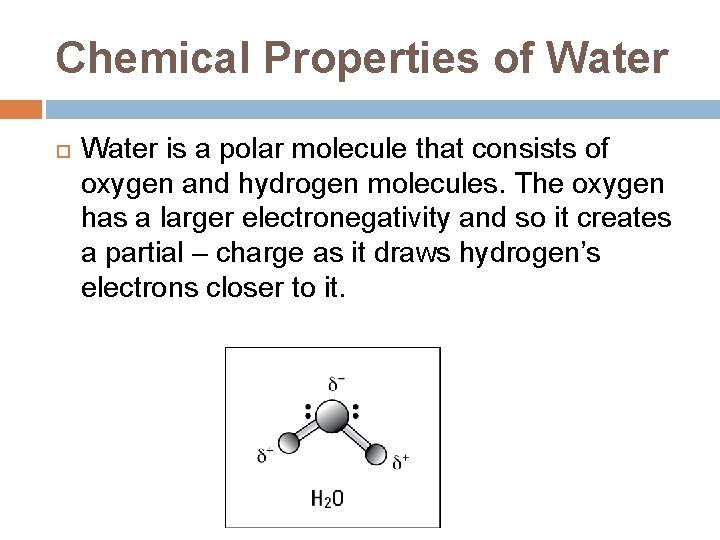
Chemical Properties of Water is a polar molecule that consists of oxygen and hydrogen molecules. The oxygen has a larger electronegativity and so it creates a partial – charge as it draws hydrogen’s electrons closer to it.

Chemical Properties of Water The large difference in charge allows for Hydrogen bonding to occur between molecules. This provides water with its unique surface tension, high boiling point and ice that is less dense. (see page 272 fig 5 for diagram) The polarity of water also allows water to dissolve other polar molecules. Like dissolves Like

Hard Water that contains dissolved calcium, magnesium and iron ions. When slightly acidic water flows through limestone, calcium, iron, magnesium and manganese irons dissolve in the water at a higher concentration.

Hard water also can deteriorate appliances that heat up water by forming scale deposits on them that prevent the water from contacting the element which decreases the efficiency of the appliance.

What’s in Polluted Water? As the amount of human activity increases the amount of contaminants found in nearby water also increase. Contaminants are classified into three types.

1. Physical Contaminants Objects that do not dissolve in water. Ex – oil, plastic, tree branches, leaves, peat, silt. The removal of physical contaminants is the first step in water purification. Most physical contaminants can easily be removed because they retain their physical properties which allow them to be physically removed by filters. Chemical means can also be used to remove the contaminants by forming precipitates which can then be filtered off.

2. Biological Contaminants Biological contaminants include bacteria and viruses which may make the water unsafe to consume. These contaminants can be removed by various methods such as UV light, filters, other bacteria and chemical means that can kill the contaminants.

3. Chemical Contaminants Chemicals that are soluble in water. Ex – metal ions, pesticides, fertilizers. Chemicals that are sprayed, thrown out and spilled contaminate the water. Some of these contaminants can kill things directly while others like phosphorus can cause algae blooms which consume all of the dissolved oxygen and make the water unsafe to use.

Arrhenius Model of Acids and Bases The classical, or Arrhenius, model was developed by Svante Arrhenius in the nineteenth century. He defined an acid as any substance that liberates or yields hydrogen ions (H+) or protons in water.

Arrhenius Model of Acids and Bases H+ ions are really just a short form for Hydronium ions which are a water molecule with a hydrogen ion bonded to it. (H 3 O+)

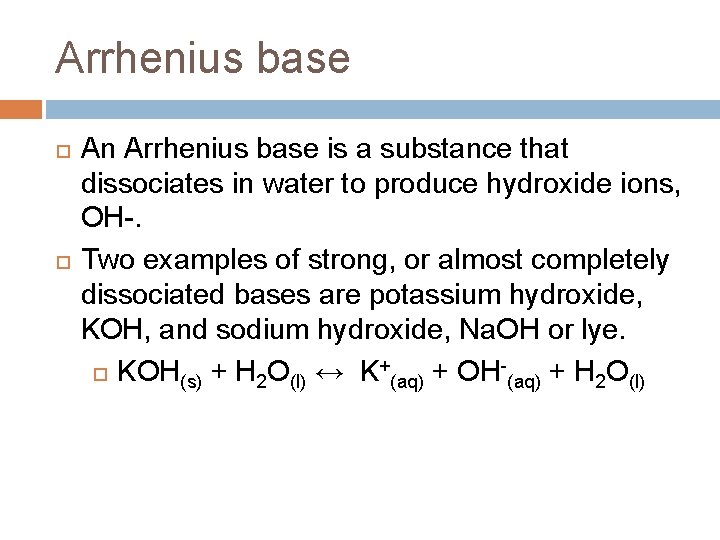
Arrhenius base An Arrhenius base is a substance that dissociates in water to produce hydroxide ions, OH-. Two examples of strong, or almost completely dissociated bases are potassium hydroxide, KOH, and sodium hydroxide, Na. OH or lye. KOH(s) + H 2 O(l) ↔ K+(aq) + OH-(aq) + H 2 O(l)

Most solutions formed by the reaction of polar molecular compounds with water are observed to have either acidic or basic properties.

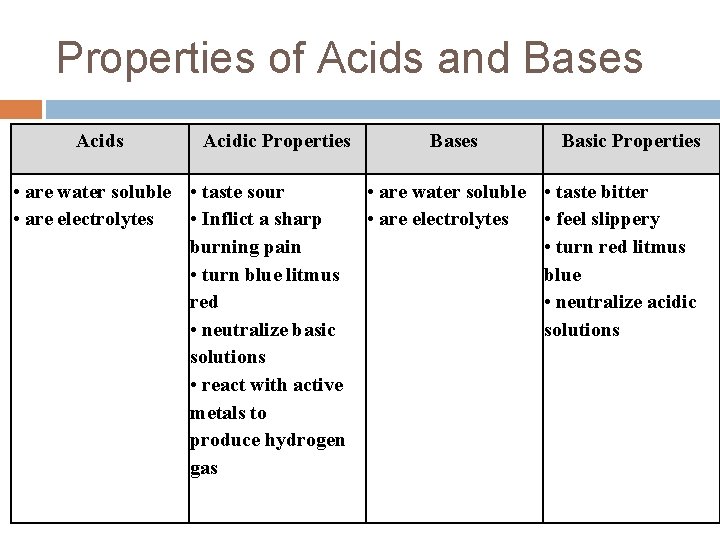
Properties of Acids and Bases Acidic Properties • are water soluble • taste sour • are electrolytes • Inflict a sharp burning pain • turn blue litmus red • neutralize basic solutions • react with active metals to produce hydrogen gas Bases Basic Properties • are water soluble • taste bitter • are electrolytes • feel slippery • turn red litmus blue • neutralize acidic solutions

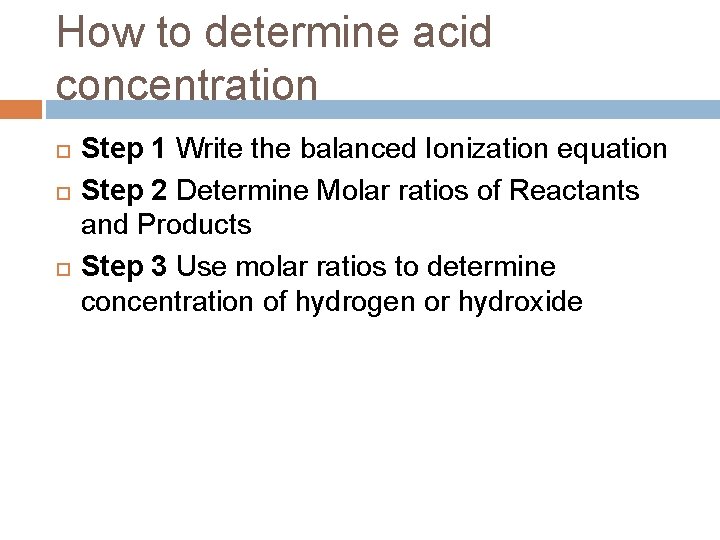
How to determine acid concentration Step 1 Write the balanced Ionization equation Step 2 Determine Molar ratios of Reactants and Products Step 3 Use molar ratios to determine concentration of hydrogen or hydroxide

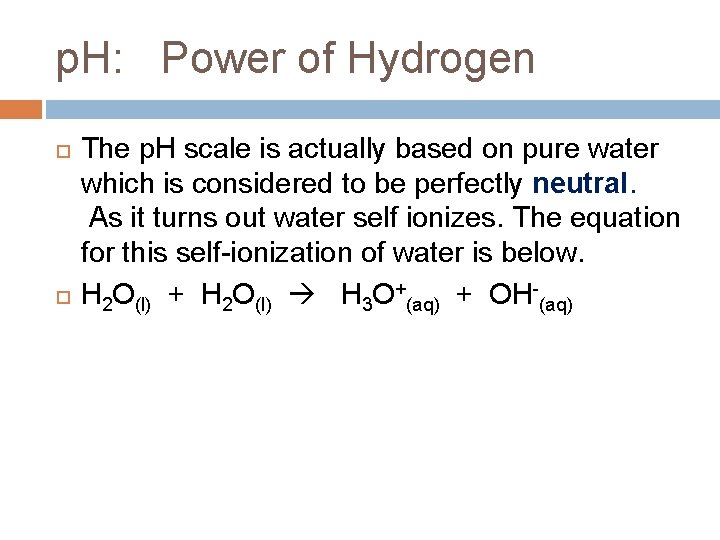
p. H: Power of Hydrogen The p. H scale is actually based on pure water which is considered to be perfectly neutral. As it turns out water self ionizes. The equation for this self-ionization of water is below. H 2 O(l) + H 2 O(l) H 3 O+(aq) + OH-(aq)

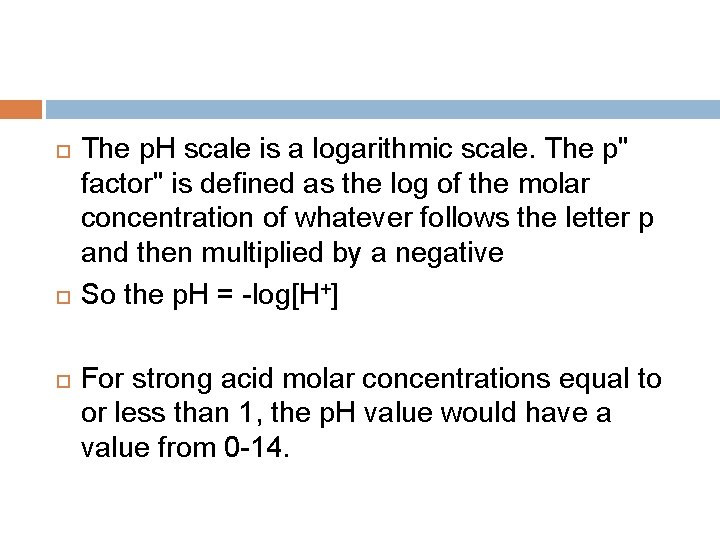
The p. H scale is a logarithmic scale. The p" factor" is defined as the log of the molar concentration of whatever follows the letter p and then multiplied by a negative So the p. H = -log[H+] For strong acid molar concentrations equal to or less than 1, the p. H value would have a value from 0 -14.

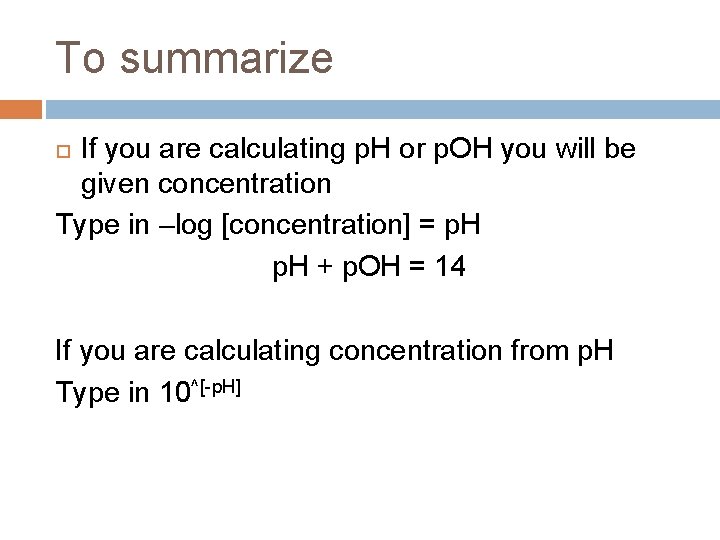
To summarize If you are calculating p. H or p. OH you will be given concentration Type in –log [concentration] = p. H + p. OH = 14 If you are calculating concentration from p. H Type in 10^[-p. H]

Acid and Bases Reactions (316321) Acids and bases have a number of characteristic chemical reactions. 1. Acids react with active metals to produce hydrogen gas and a salt of the metal and acid. Fe(s) + 2 HCl(aq) --> H 2(g) + Fe. Cl 2(aq)

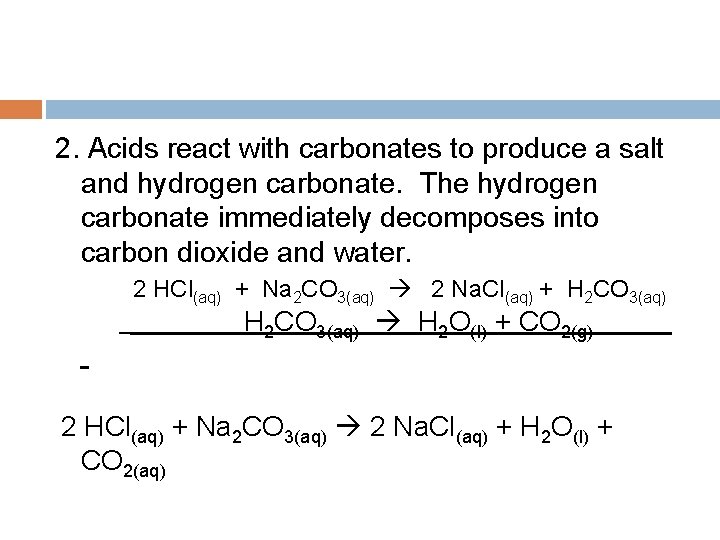
2. Acids react with carbonates to produce a salt and hydrogen carbonate. The hydrogen carbonate immediately decomposes into carbon dioxide and water. 2 HCl(aq) + Na 2 CO 3(aq) 2 Na. Cl(aq) + H 2 CO 3(aq) H 2 O(l) + CO 2(g) 2 HCl(aq) + Na 2 CO 3(aq) 2 Na. Cl(aq) + H 2 O(l) + CO 2(aq)

3. Acids react with a base to form salt and water. When an acid and a base of equal strength are mixed they react to form products that have a p. H of near or at 7, this is defined as a neutralization reaction. A Neutralization reaction always produces a salt and water.

The products of a neutralization reaction of an acid and a base are salt and water. Acid + Base Salt + water

ACID BASE TITRATIONS

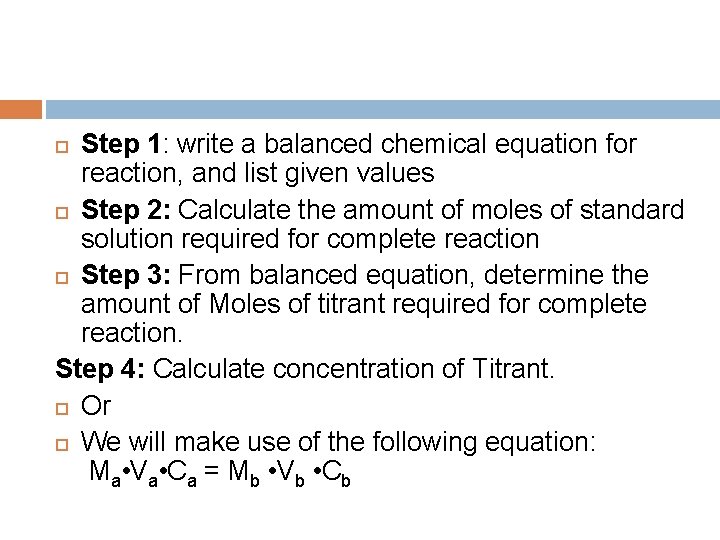
Step 1: write a balanced chemical equation for reaction, and list given values Step 2: Calculate the amount of moles of standard solution required for complete reaction Step 3: From balanced equation, determine the amount of Moles of titrant required for complete reaction. Step 4: Calculate concentration of Titrant. Or We will make use of the following equation: Ma • Va • Ca = Mb • Vb • Cb

BOYLE’S LAW

Boyle’s Law Boyle used the manometer and barometer to study the pressures and volumes of different samples of different gases. The results of his studies can be summarized in a simple statement which has come to be known as Boyle's law: At any constant temperature, the product of the pressure and the volume of any size sample of any gas is a constant.

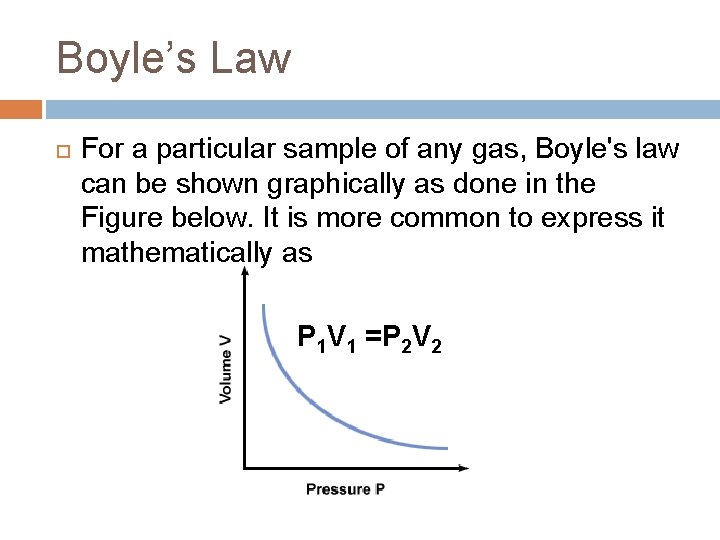
Boyle’s Law For a particular sample of any gas, Boyle's law can be shown graphically as done in the Figure below. It is more common to express it mathematically as P 1 V 1 =P 2 V 2

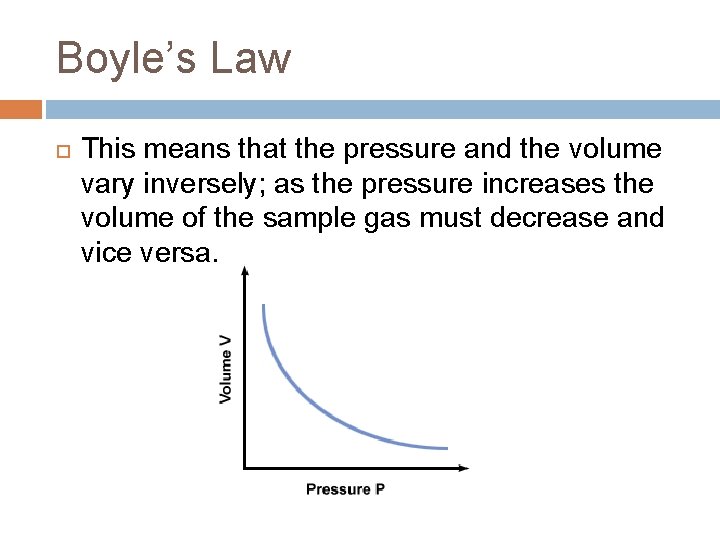
Boyle’s Law This means that the pressure and the volume vary inversely; as the pressure increases the volume of the sample gas must decrease and vice versa.

CHARLES LAW: THE RELATIONSHIP BETWEEN THE VOLUME AND TEMPERATURE

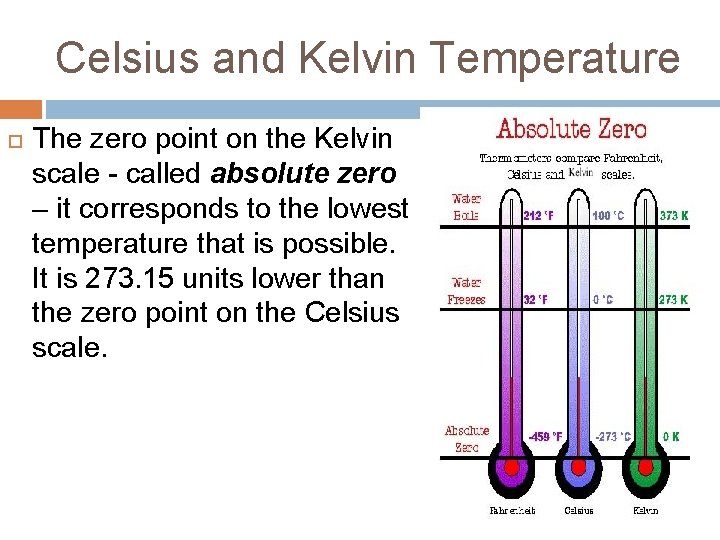
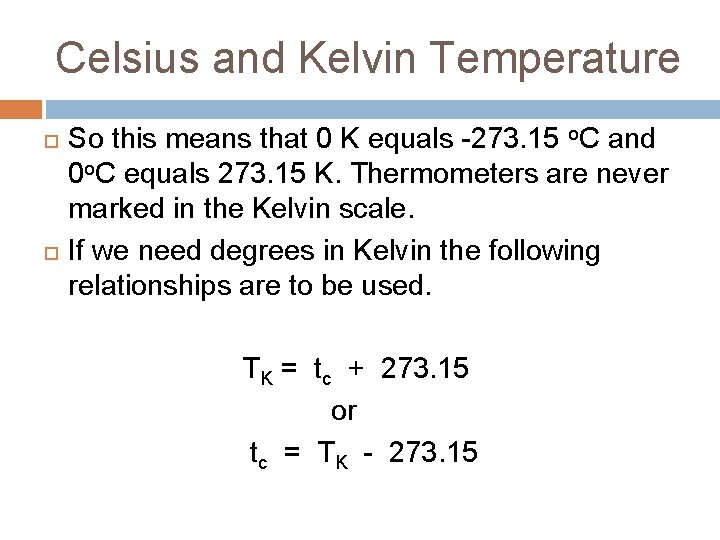
Celsius and Kelvin Temperature The zero point on the Kelvin scale - called absolute zero – it corresponds to the lowest temperature that is possible. It is 273. 15 units lower than the zero point on the Celsius scale.

Celsius and Kelvin Temperature So this means that 0 K equals -273. 15 o. C and 0 o. C equals 273. 15 K. Thermometers are never marked in the Kelvin scale. If we need degrees in Kelvin the following relationships are to be used. TK = tc + 273. 15 or tc = TK - 273. 15

CHARLES LAW

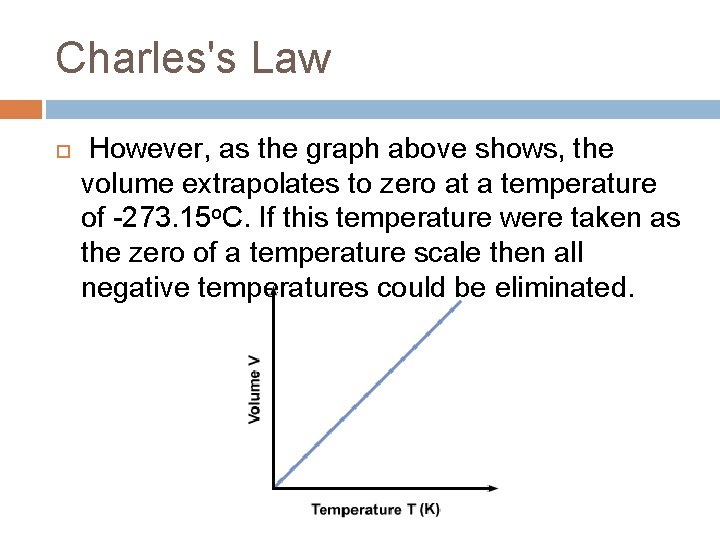
Charles Law The direct relationship between the volume of a gas and the temperature of the gas (on the Kelvin temperature scale) is known as Charles Law. According to this law, as the temperature of a gas increases, the volume increases proportionally, provided that the pressure and the amount of gas remains the same.

Charles's Law However, as the graph above shows, the volume extrapolates to zero at a temperature of -273. 15 o. C. If this temperature were taken as the zero of a temperature scale then all negative temperatures could be eliminated.

Charles's Law Such a temperature scale is now the fundamental scale of temperature in the SI. It is called the absolute scale, thermodynamic scale, and the Kelvin scale. Temperature on the Kelvin scale, and only on the Kelvin scale, is symbolized by T.

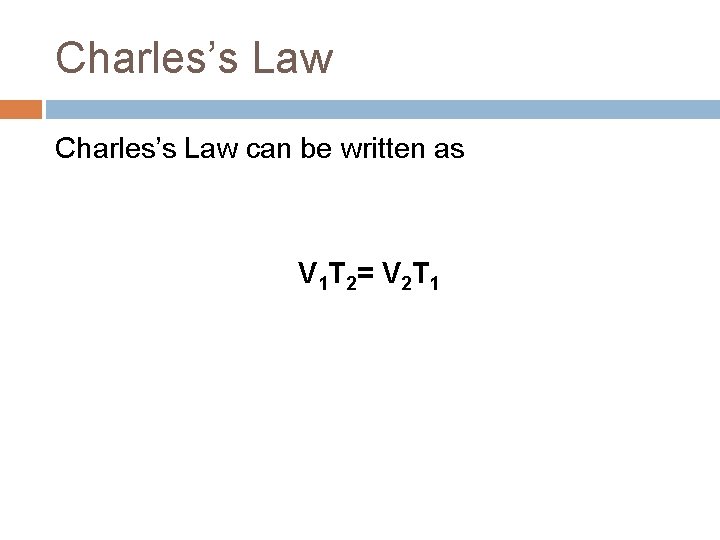
Charles’s Law can be written as V 1 T 2= V 2 T 1

DALTON’S LAW OF PARTIAL PRESSURES

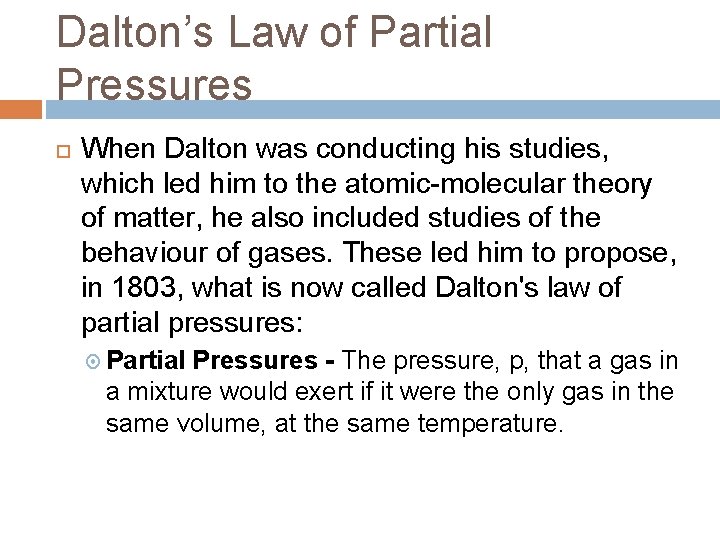
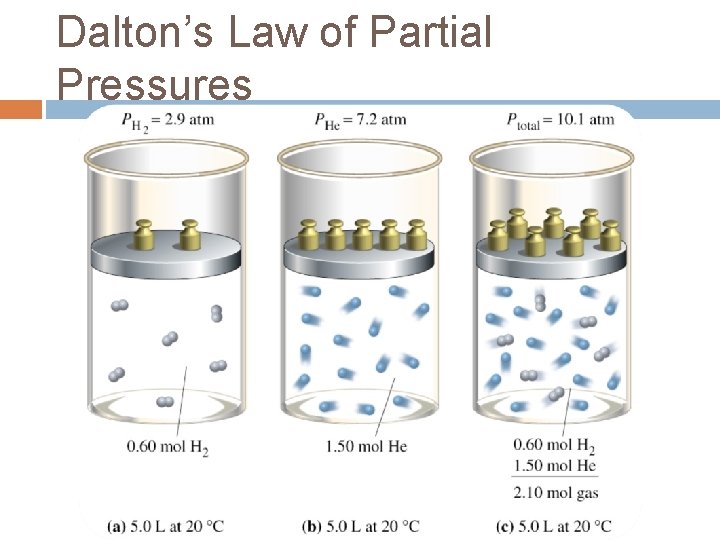
Dalton’s Law of Partial Pressures When Dalton was conducting his studies, which led him to the atomic-molecular theory of matter, he also included studies of the behaviour of gases. These led him to propose, in 1803, what is now called Dalton's law of partial pressures: Partial Pressures - The pressure, p, that a gas in a mixture would exert if it were the only gas in the same volume, at the same temperature.

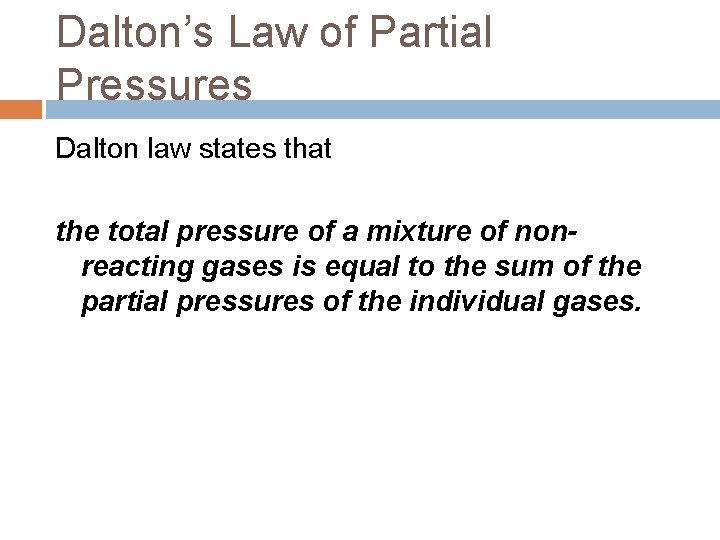
Dalton’s Law of Partial Pressures Dalton law states that the total pressure of a mixture of nonreacting gases is equal to the sum of the partial pressures of the individual gases.

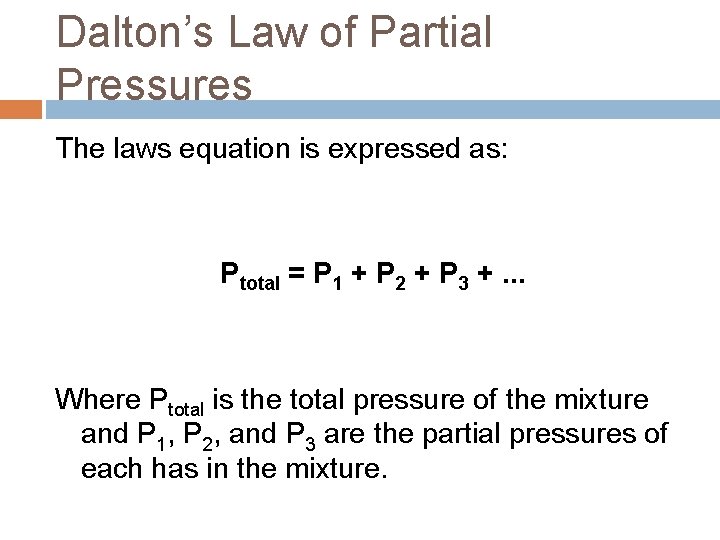
Dalton’s Law of Partial Pressures The laws equation is expressed as: Ptotal = P 1 + P 2 + P 3 +. . . Where Ptotal is the total pressure of the mixture and P 1, P 2, and P 3 are the partial pressures of each has in the mixture.

Dalton’s Law of Partial Pressures
 Water and water and water water
Water and water and water water Physical properties of a pure substance
Physical properties of a pure substance Unit 6 review questions
Unit 6 review questions Chemical and physical properties
Chemical and physical properties Characteristics of pure water
Characteristics of pure water Water chemical and physical properties
Water chemical and physical properties Unit 11 water water everywhere
Unit 11 water water everywhere Properties of pure substances
Properties of pure substances Pure substance examples pictures
Pure substance examples pictures Pure substance properties
Pure substance properties Volumetric properties of pure fluids
Volumetric properties of pure fluids Properties of pure substance
Properties of pure substance Properties of pure substances thermodynamics
Properties of pure substances thermodynamics Unit test algebra 2
Unit test algebra 2 Nutritech beta ecdysterone
Nutritech beta ecdysterone Ventura water pure
Ventura water pure Is pure water colorless
Is pure water colorless Raoult's law and dalton's law
Raoult's law and dalton's law Purified water ip
Purified water ip Pure water
Pure water Tap water pure substance or mixture
Tap water pure substance or mixture Why is water colourless
Why is water colourless Is water a pure substance
Is water a pure substance Normal range sodium
Normal range sodium Compounds bbc bitesize
Compounds bbc bitesize Unit 4 homework 4 pure imaginary numbers
Unit 4 homework 4 pure imaginary numbers Intensive vs extensive properties
Intensive vs extensive properties Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Writ of certiorari ap gov example
Writ of certiorari ap gov example Nader amin-salehi
Nader amin-salehi Inclusion criteria examples
Inclusion criteria examples Narrative review vs systematic review
Narrative review vs systematic review Amino acid optical isomers
Amino acid optical isomers Bromine test for unsaturation
Bromine test for unsaturation Physical properties of sulphuric acid
Physical properties of sulphuric acid 2 hydrogen 1 oxygen
2 hydrogen 1 oxygen Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties 5 starch properties
5 starch properties Thermophysical properties of dental materials
Thermophysical properties of dental materials Lesson outline lesson 2 - physical properties answer key
Lesson outline lesson 2 - physical properties answer key Cardboard mechanical properties
Cardboard mechanical properties Physical property
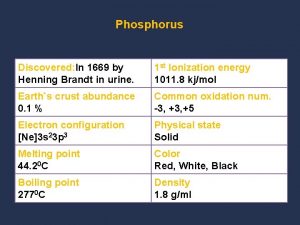
Physical property Chemical properties of phosphorus
Chemical properties of phosphorus Physical properties of paint
Physical properties of paint The physical properties of metals include luster and
The physical properties of metals include luster and