Chemistry Review Physical vs Chemical Properties Physical properties







- Slides: 23

Chemistry Review

Physical vs Chemical Properties • Physical properties describe the physical appearance and composition of a substance Ex. Boiling point, melting point, malleability, ductility, colour etc. • Chemical properties describe the ability of a substance to change into a new substance (or substances) Ex. Ability to burn, react in air or water etc.

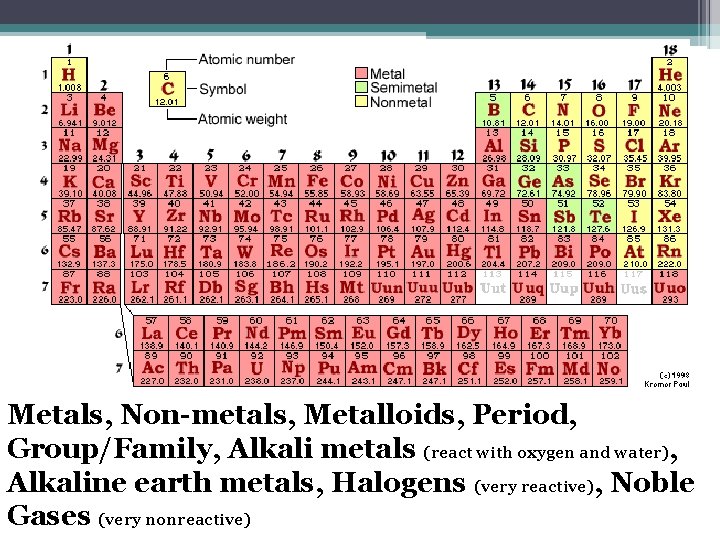
Metals, Non-metals, Metalloids, Period, Group/Family, Alkali metals (react with oxygen and water), Alkaline earth metals, Halogens (very reactive), Noble Gases (very nonreactive)

Metals, Non-metals, Metalloids • Metals : ▫ ▫ ▫ Conduct heat and electricity Ductile Malleable Shiny, often silver Solid at room temperature (except mercury) • Non-metals: ▫ Poor conductors of heat/electricity ▫ At room temperature some are solids, some gases, and Bromine is a liquid • Metalloids: ▫ Properties are intermediate of metals and non-metals

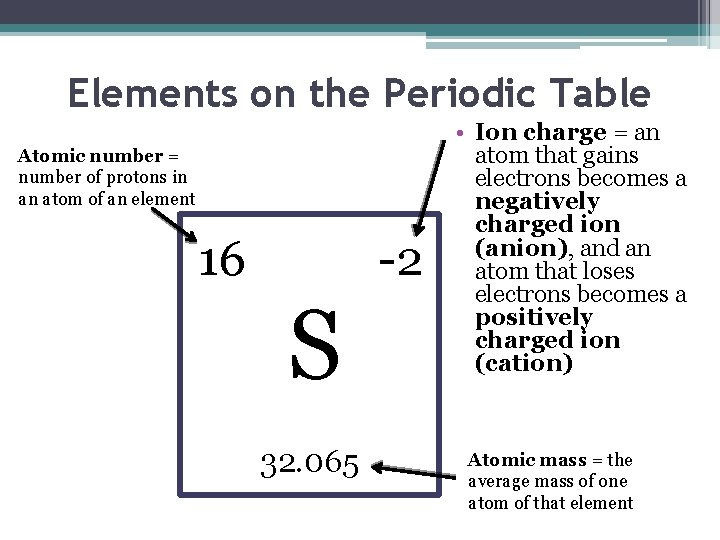
Elements on the Periodic Table Atomic number = number of protons in an atom of an element 16 -2 S 32. 065 • Ion charge = an atom that gains electrons becomes a negatively charged ion (anion), and an atom that loses electrons becomes a positively charged ion (cation) Atomic mass = the average mass of one atom of that element

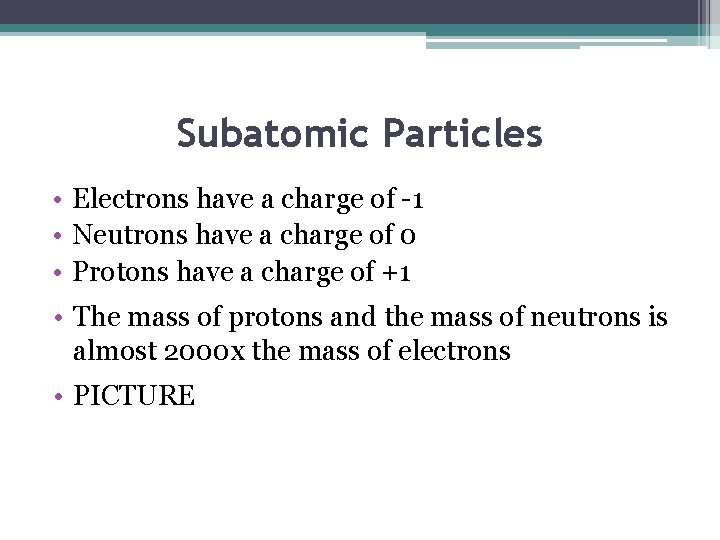
Subatomic Particles • Electrons have a charge of -1 • Neutrons have a charge of 0 • Protons have a charge of +1 • The mass of protons and the mass of neutrons is almost 2000 x the mass of electrons • PICTURE

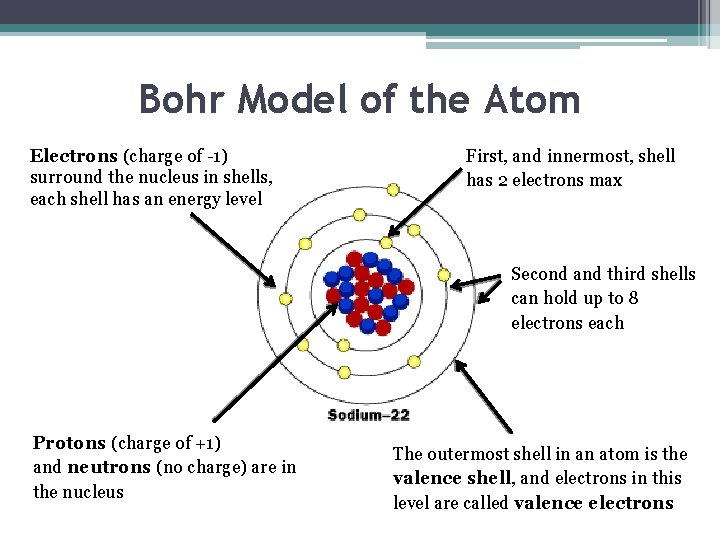
Bohr Model of the Atom Electrons (charge of -1) surround the nucleus in shells, each shell has an energy level First, and innermost, shell has 2 electrons max Second and third shells can hold up to 8 electrons each Protons (charge of +1) and neutrons (no charge) are in the nucleus The outermost shell in an atom is the valence shell, and electrons in this level are called valence electrons

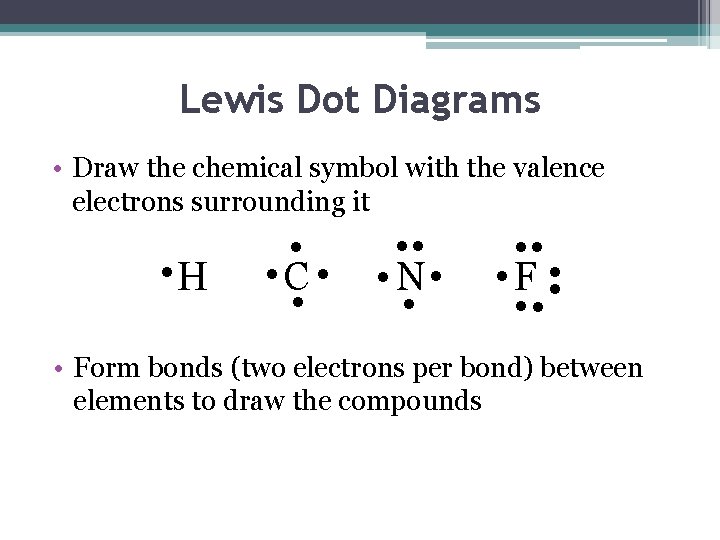
Lewis Dot Diagrams • Draw the chemical symbol with the valence electrons surrounding it • H • • C • • • N • • • F • • • Form bonds (two electrons per bond) between elements to draw the compounds

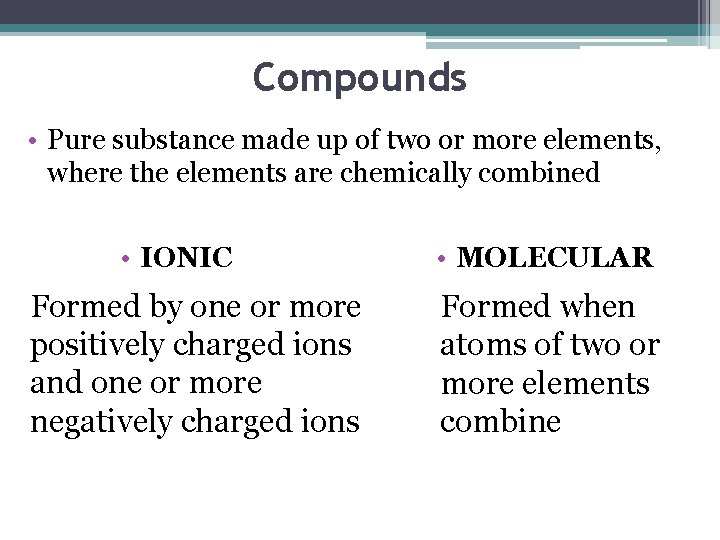
Compounds • Pure substance made up of two or more elements, where the elements are chemically combined • IONIC Formed by one or more positively charged ions and one or more negatively charged ions • MOLECULAR Formed when atoms of two or more elements combine

Ionic Compounds • Electrons are transferred between the atoms that are combining • Metal atoms tend to lose electrons, non-metal atoms tend to gain electrons • Bonded ions will now have 8 electrons in their valence shells • Name metal ion first, name non-metal ion with “ide” when it becomes a negative ion

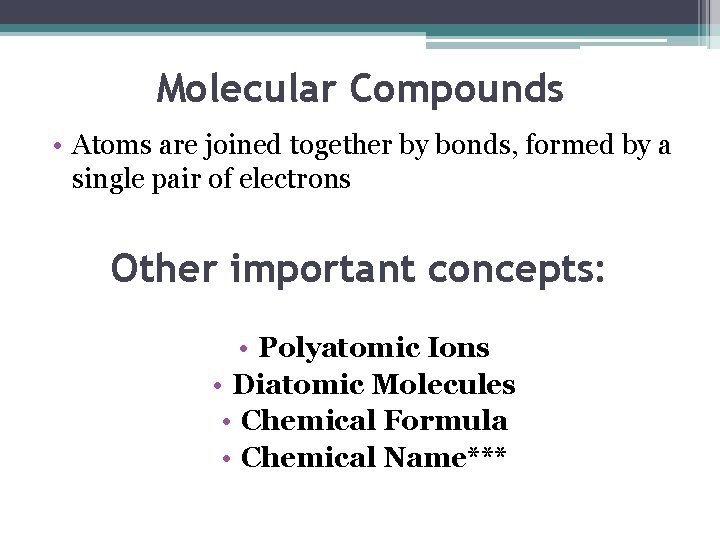
Molecular Compounds • Atoms are joined together by bonds, formed by a single pair of electrons Other important concepts: • Polyatomic Ions • Diatomic Molecules • Chemical Formula • Chemical Name***

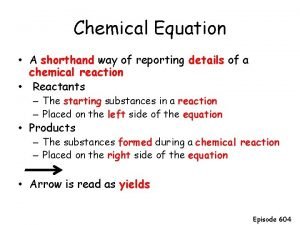
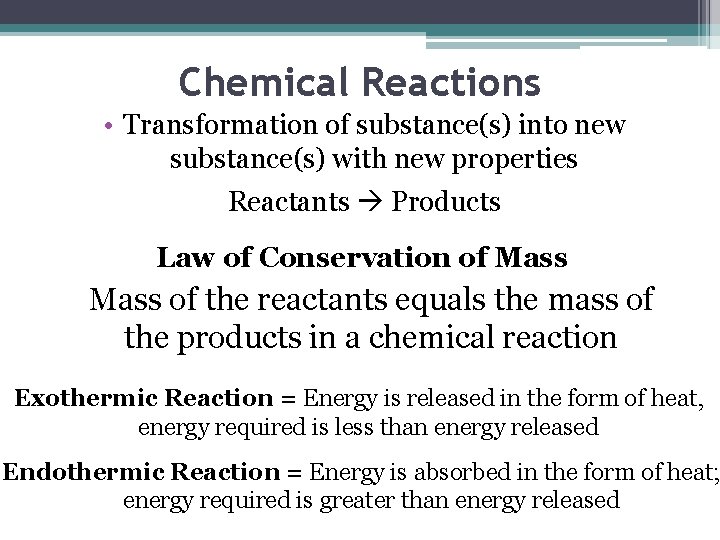
Chemical Reactions • Transformation of substance(s) into new substance(s) with new properties Reactants Products Law of Conservation of Mass of the reactants equals the mass of the products in a chemical reaction Exothermic Reaction = Energy is released in the form of heat, energy required is less than energy released Endothermic Reaction = Energy is absorbed in the form of heat; energy required is greater than energy released

• Word Equation vs Formula Equation

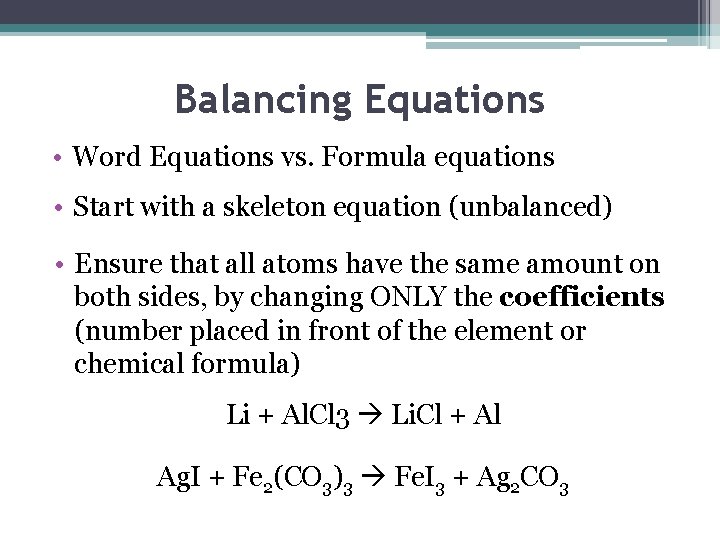
Balancing Equations • Word Equations vs. Formula equations • Start with a skeleton equation (unbalanced) • Ensure that all atoms have the same amount on both sides, by changing ONLY the coefficients (number placed in front of the element or chemical formula) Li + Al. Cl 3 Li. Cl + Al Ag. I + Fe 2(CO 3)3 Fe. I 3 + Ag 2 CO 3

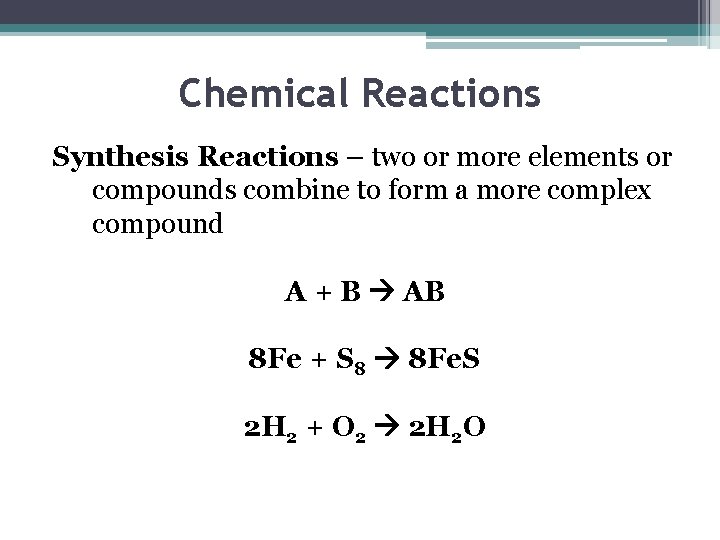
Chemical Reactions Synthesis Reactions – two or more elements or compounds combine to form a more complex compound A + B AB 8 Fe + S 8 8 Fe. S 2 H 2 + O 2 2 H 2 O

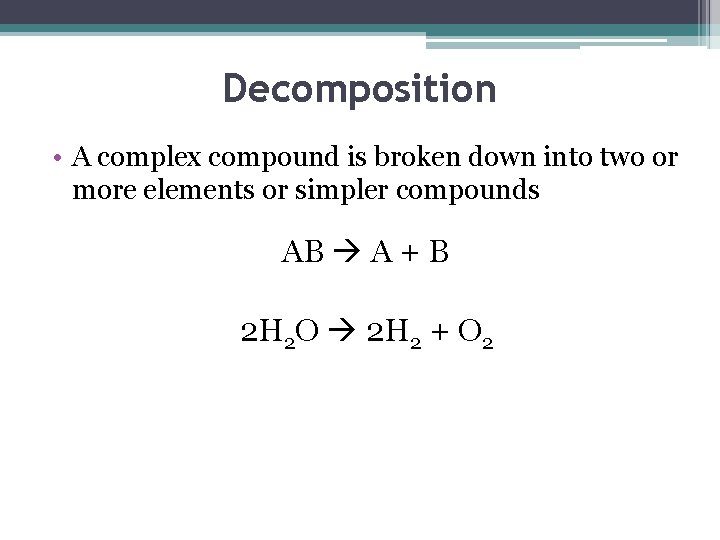
Decomposition • A complex compound is broken down into two or more elements or simpler compounds AB A + B 2 H 2 O 2 H 2 + O 2

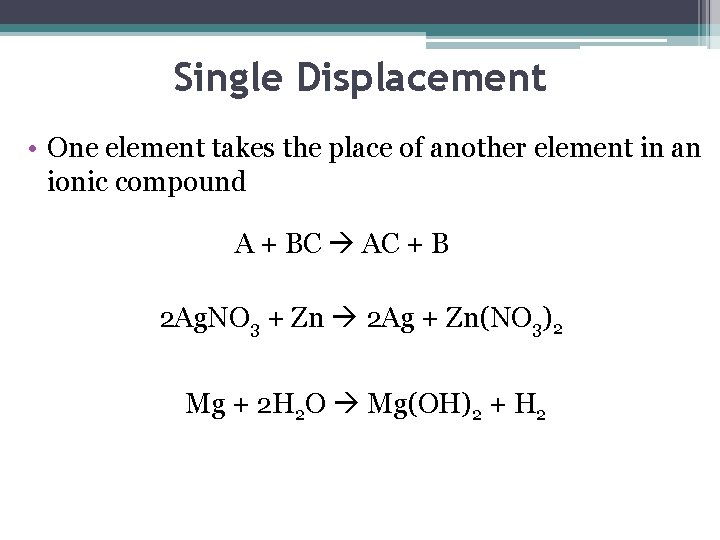
Single Displacement • One element takes the place of another element in an ionic compound A + BC AC + B 2 Ag. NO 3 + Zn 2 Ag + Zn(NO 3)2 Mg + 2 H 2 O Mg(OH)2 + H 2

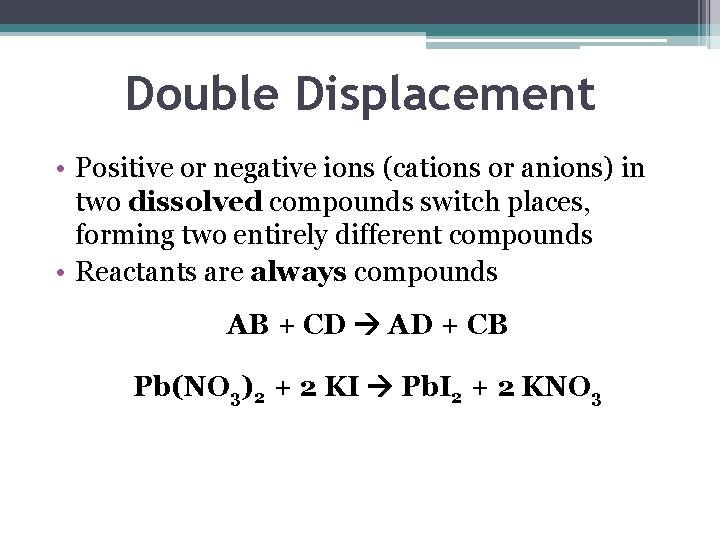
Double Displacement • Positive or negative ions (cations or anions) in two dissolved compounds switch places, forming two entirely different compounds • Reactants are always compounds AB + CD AD + CB Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3

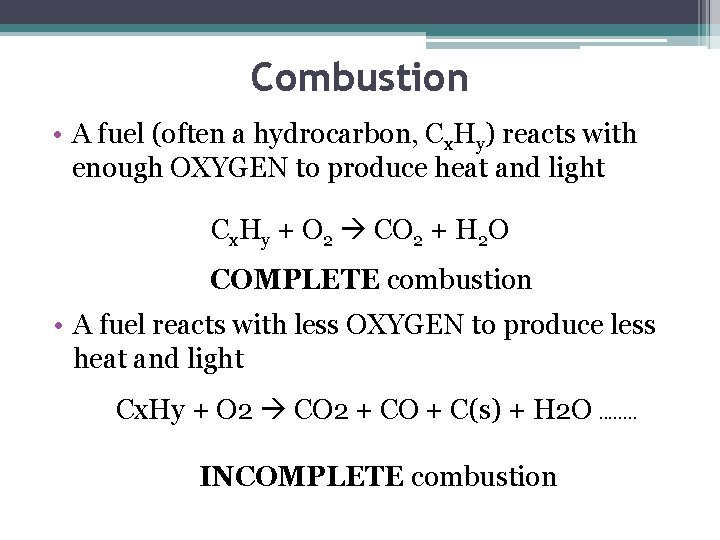
Combustion • A fuel (often a hydrocarbon, Cx. Hy) reacts with enough OXYGEN to produce heat and light Cx. Hy + O 2 CO 2 + H 2 O COMPLETE combustion • A fuel reacts with less OXYGEN to produce less heat and light Cx. Hy + O 2 CO 2 + CO + C(s) + H 2 O. . . . INCOMPLETE combustion

Acids • Proton donors • Release H+ ions in aqueous solutions • High concentration of H+ ions • p. H less than 7 • Taste sour • Turn blue litmus paper re. D • Conduct electricity, dissolve in water (aqueous solution), cause skin burns or irritation

Bases • Proton acceptors • Release OH- ions in aqueous solutions • High concentration of OH- ions (low H+) • p. H greater than 7 • Taste bitter • Turn red litmus paper Blue • Conduct electricity, dissolve in water (aqueous solution), cause skin burns or irritation

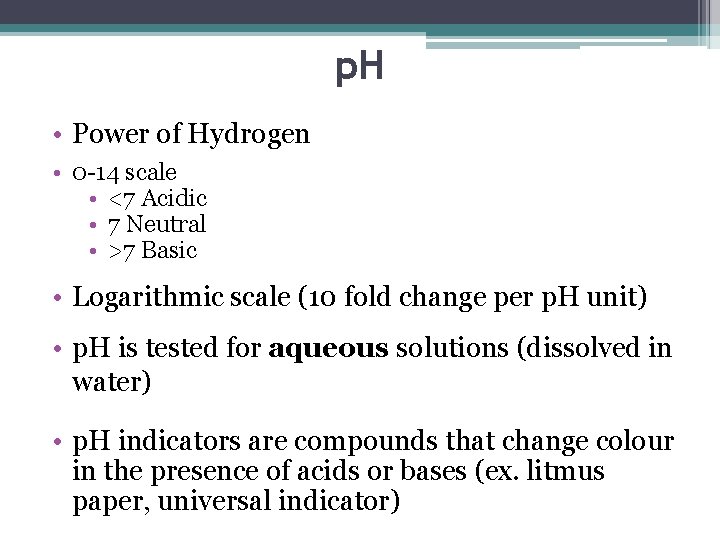
p. H • Power of Hydrogen • 0 -14 scale • <7 Acidic • 7 Neutral • >7 Basic • Logarithmic scale (10 fold change per p. H unit) • p. H is tested for aqueous solutions (dissolved in water) • p. H indicators are compounds that change colour in the presence of acids or bases (ex. litmus paper, universal indicator)

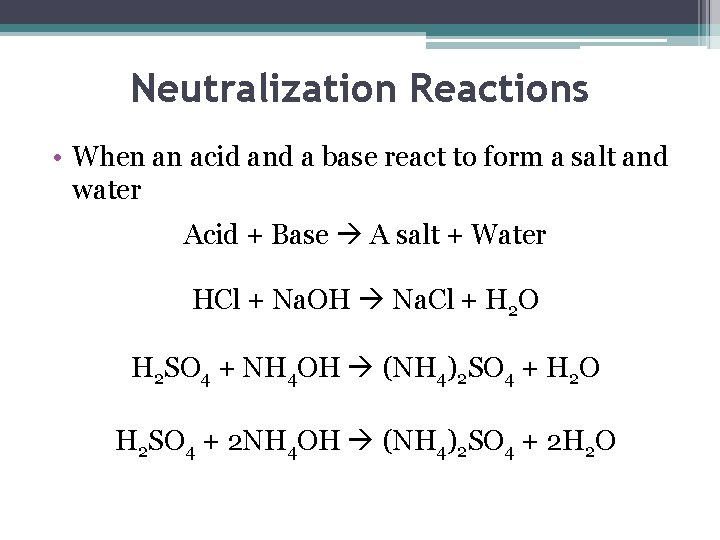
Neutralization Reactions • When an acid and a base react to form a salt and water Acid + Base A salt + Water HCl + Na. OH Na. Cl + H 2 O H 2 SO 4 + NH 4 OH (NH 4)2 SO 4 + H 2 O H 2 SO 4 + 2 NH 4 OH (NH 4)2 SO 4 + 2 H 2 O
 Physical properties and chemical properties
Physical properties and chemical properties Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Empirical formula pogil
Empirical formula pogil Chemical formula of love
Chemical formula of love Chemical properties of sulphuric acid
Chemical properties of sulphuric acid Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties Chemical properties of dental materials
Chemical properties of dental materials Physical properties
Physical properties Hardness property of matter
Hardness property of matter Physical properties of oxygen family
Physical properties of oxygen family Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Physical and chemical properties interactive
Physical and chemical properties interactive Physical and chemical properties of silver
Physical and chemical properties of silver Lactose is made up of
Lactose is made up of Physical property
Physical property Water chemical and physical properties
Water chemical and physical properties Physical and chemical properties sorting activity
Physical and chemical properties sorting activity Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Molekülerite nedir
Molekülerite nedir Pixl knowit gcse chemistry chemical changes
Pixl knowit gcse chemistry chemical changes Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 Chemist shorthand way of representing chemical reaction
Chemist shorthand way of representing chemical reaction Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key