Physical Properties vs Chemical Properties What are chemical



















- Slides: 21

Physical Properties vs. Chemical Properties

What are chemical properties of matter? During CHEMICAL REACTIONS, atoms rearrange to form products with different properties. CHEMICAL PROPERTIES are characteristics of an element or compound in chemical reactions. In a chemical change, the substances change chemically and show different properties after the change. Differences in chemical and physical properties of a substance are used to separate mixtures and tell the difference between compounds.

What are physical properties of matter? In a physical change, the substances are not altered chemically, but merely change to another state of matter or they simply separate and combine. PHYSICAL PROPERTIES are characteristics of an element or compound that can be seen without any chemical reaction of the substance. They’re the properties the element or compound already has. Color, density, mass, and solubility are all physical properties.

Physical & Chemical Changes When a physical change occurs, it means that the substance changes its appearance but not its molecular identity or structure. Examples of physical changes include: Ë Shredding a piece of paper Ë Drawing metal into a wire (copper) Ë Pounding metal into thin sheets (aluminum) Ë Breaking a sheet of glass Ë Filtering a solid from a liquid Ë A liquid expanding as it is heated (boiling water)

Chemical Changes A chemical change occurs when a substance changes its identity (molecular structure). For example – when copper metal is placed in nitric acid solution, it reacts with the acid. The copper metal changes its molecular identity when it combines with the nitric acid and it becomes copper nitrate.

Chemical changes occur because chemical reactions have taken place. Examples of chemical changes include – Ë Silver metal reacting with sulfur to form sulfur oxide Ë Burning hydrogen gas in air Ë Heating a compound until it breaks down or decomposes Ë The oxidation of metals in air (rust) Ë The reaction of an acid and a base

These are all examples of chemical changes because a reaction has taken place, and the molecular identity of the substance has changed. In each case, the atoms of the reactants have not changed. Reactants are any substance that takes part in chemical reactions. However, the atoms have combined in new ways to form different products with different molecules. This shows that chemical changes cause changes in matter. There are several common signs that show when a chemical change has occurred. Ë Ë Ë One is that there is a change in temperature, since reactions always either produce or absorb heat. Another is that the substance or solution changes color. And another is the production of a solid precipitate or the development of a gas. In every case, a chemical change has occurred if the identity (molecular structure) of a substance has changed. If there has not been a change in identity, then only physical changes have occurred.

Law of Conservation of Matter The LAW OF CONSERVATION OF MATTER states that the amount of matter remains constant even if a change has occurred. This means that even though an object may have undergone a physical or chemical change, the amount of matter (i. e. the mass) must ALWAYS remain the same. What does this mean to you?

A closer look at Physical Properties Some physical properties are dependent on the sample size; Ë Mass Ë Weight Ë Volume Some physical properties are not dependent on sample size: Density Ë Ë Melting point Ë Boiling point Ë Ë Solubility Ability to attract a magnet Ë State of matter Ë color

Size Dependent Properties Some physical properties depend on the size of an object. Suppose you need to move a box. The size of the box would be important in deciding if you need to use your backpack or a truck. You begin measuring the width, height, and depth of the box. If you multiply them together, you calculated the box’s VOLUME = Width x Height x Depth (or length x width x height) The VOLUME of an object is the amount of space it occupies.

Mass Another physical property that depends on the size of the object is mass. MASS is the measurement of how much matter an object contains. A bowling ball has more mass than a basketball. Weight is different from mass. Weight is a measurement of force; it depends on the mass of the object and the pull of gravity on that mass. If you were to travel to other planets, your weight would change but your size and mass would not!

Size Independent Properties Another physical property, density, does not depend on the size of an object. DENSITY is how much mass a material has per unit of volume. Denser materials have more atoms in a given space than less dense materials. DENSITY is found by dividing the mass of an object by its volume. Density = Mass ÷ Volume (or Mass ÷ (length X width x height))

The density of water is the same in a glass as is it in a tub. The density of an object will change, however, if the mass changes and the volume remains the same. Another property, solubility, also does not depend on the size of the object. SOLUBILITY is the number of grams of one substance that will dissolve in 100 g of another substance at a given temperature. The amount of drink mix that can be dissolved in 100 g of water is the same in a pitcher as it is when it is poured into a glass.

Displacement DISPLACEMENT reactions occur when one element replaces a similar element in a compound. For example – if you place an iron nail into a beaker of copper (II) chloride solution, you will begin to see reddish copper forming on the iron nail. In this reaction, iron replaces copper in the solution and the copper falls out of the solution as a metal. A SOLUTION is a mixture of two or more substances that is uniform at the molecular level. Uniform means there are no clumps bigger than a molecule and the solution has the same ingredients everywhere.

Melting Point, Boiling Point, & Freezing Point Melting point and boiling point also do not depend upon an object’s size. The temperature at which a solid changes into a liquid is called its MELTING POINT. The temperature at which a liquid changes form a liquid into a gas is called its BOILING POINT. The temperature at which a liquid becomes a solid is called its FREEZING POINT.

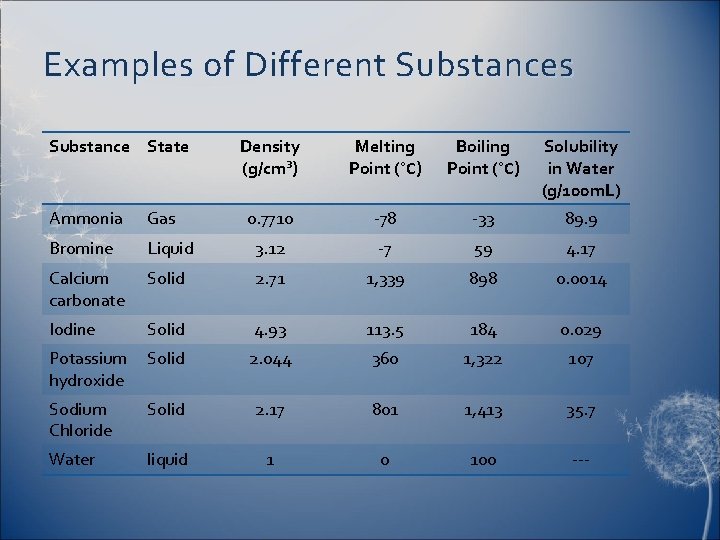
Examples of Different Substances Substance State Density (g/cm³) Melting Point (°C) Boiling Point (°C) Solubility in Water (g/100 m. L) 0. 7710 -78 -33 89. 9 Ammonia Gas Bromine Liquid 3. 12 -7 59 4. 17 Calcium carbonate Solid 2. 71 1, 339 898 0. 0014 Iodine Solid 4. 93 113. 5 184 0. 029 Potassium hydroxide Solid 2. 044 360 1, 322 107 Sodium Chloride Solid 2. 17 801 1, 413 35. 7 Water liquid 1 0 100 ---

Changing States in Matter Another common physical change occurs when matter changes from one state to another. Ice melting to become liquid water. Matter can change from one state to another. Freezing is the opposite of melting. During freezing, a liquid becomes a solid. A liquid can also turn into a gas; this is called EVAPORATION. The reverse of this process is called CONDENSATION – a gas changes to a liquid.

In some cases, matter changes between solid and gas states without ever becoming a liquid. The process in which a solid becomes a gas by skipping the liquid state is called SUBLIMATION. The opposite of this process, where a gas becomes a solid by skipping the liquid state is called deposition.

How is matter recycled in the universe? The Law of Conservation of Mass states that matter cannot be created or lost in a physical change or a chemical reaction. In other words, the total amount of the reactants (mass of the reactants) must equal the total amount of matter of the products (mass of the products) in a chemical reaction. Reactants are any substances that take part in chemical reactions.

Matter cannot be created or lost, but it can change forms. For example – if you burned charcoal, the charcoal would undergo a combustion reaction. If you added the masses of ALL the products of the reaction (ashes, soot, gases), however, you would find that this mass would be equal to the original mass of the charcoal. Mass was not created or lost, it just changed forms: From charcoal to ashes, soot, and gases. So again, how is matter recycled in the universe? So , can we ever make matter go away?

Summing it all up Based on the information we learned in the last unit about elements, compounds, and mixtures AND the information we learned about physical and chemical properties I want you to answer the following question on your own paper (not your notes) and have it ready to turn in at the end of class – How can permanent atoms make temporary substances?