Treatment of Cutaneous TCell Lymphoma Focus on RelapsedRefractory




























- Slides: 53

Treatment of Cutaneous T-Cell Lymphoma: Focus on Relapsed/Refractory Disease This program is supported by an educational grant from Ligand Clinical Care Options, LLC

Treatment of Cutaneous T-Cell Lymphoma: Focus on Relapsed/Refractory Disease Pathophysiology and Treatment John A. Zic, MD Vanderbilt University Medical Center Nashville, Tennessee Diagnosis and Staging Timothy M. Kuzel, MD Northwestern University Chicago, Illinois

Focus on Relapsed/Refractory Disease First Description of Mycosis Fungoides clinicaloptions. com/oncology

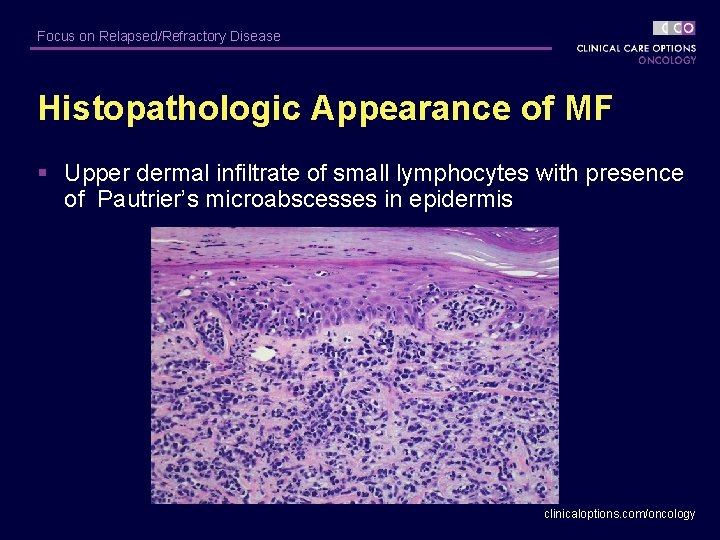
Focus on Relapsed/Refractory Disease Primary Cutaneous T-Cell Lymphoma (CTCL) § Mycosis fungoides (MF) – Most common T-Cell lymphoma of the skin – Isolated patches, plaques, and/or tumors § Classic histopathologic features – Superficial dermal infiltrate of malignant lymphocytes – Cerebriform nuclei within malignant lymphocytes – Epidermotropism (Pautrier’s microabscesses) – Early stage lesions include reactive benign T-lymphocytes clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Histopathologic Appearance of MF § Upper dermal infiltrate of small lymphocytes with presence of Pautrier’s microabscesses in epidermis clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease TNM and B Staging of MF* § T (Skin) § M (Viscera) – T 1 Limited patch/plaque (< 10% BSA) – M 0 No visceral involvement – T 2 Generalized patch/plaque – M 1 Visceral involvement – T 3 Tumors § B (Blood) – T 4 Generalized erythroderma * Staging should be done at diagnosis only and does not change with treatment effect; provides prognostic information for patients. – B 0 Atypical circulating cells not present (< 5%) – B 1 > 5% atypical cells, < 1000 atypical cells/mm 3 – B 2 > 1000 atypical cells/mm 3 clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease TNM and B Staging of MF (continued) § N (Nodes) – N 0 No clinically abnormal nodes – N 1 Clinical abnormal node – N 2 Biopsy performed, not involved with MF – N 3 Biopsy performed, involved with MF – LN 3 large clusters of atypical cells (> 6 cells/cluster) to partial effacement – LN 4 lymph node effacement – LN 0 normal; LN 1 dermatopathic reactive lymph node – LN 2 dermatopathic node with atypical cells (< 6 cells/cluster) clinicaloptions. com/oncology

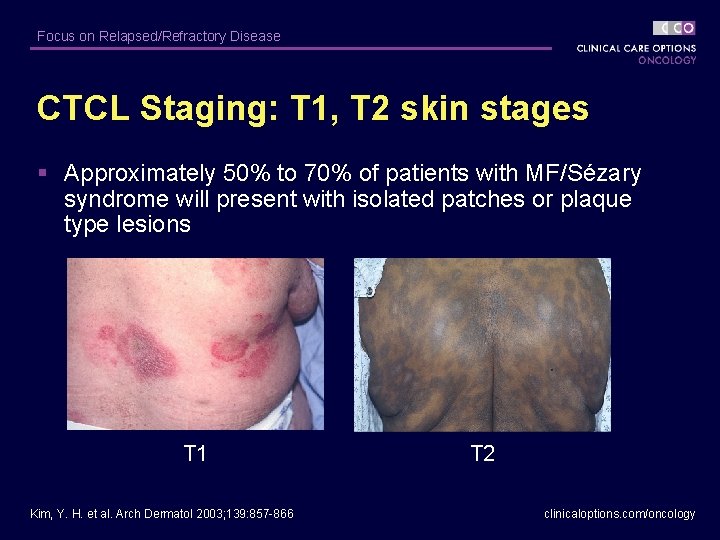
Focus on Relapsed/Refractory Disease CTCL Staging: T 1, T 2 skin stages § Approximately 50% to 70% of patients with MF/Sézary syndrome will present with isolated patches or plaque type lesions T 1 Kim, Y. H. et al. Arch Dermatol 2003; 139: 857 -866 T 2 clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL Staging: T 3 Tumor Stage § Tumors commonly observed with progression from earlier stages – Rarely present at initial diagnosis clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL Staging: T 4 Skin Stage § MF/Sézary syndrome; generalized erythroderma § Exfoliative erythroderma – Significant peripheral blood involvement in > 90% of patients with erythroderma – Disabling pruritus Sausville EA, et al. Ann Intern Med. 1988; 109: 372 -382. Schester GP, et al. Blood. 1987; 69: 1 -9. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL Staging: T 4 Skin Stage (continued) § Poor prognostic features – PAS-positive cytoplasmic inclusions – CD 4 positive, CD 7 negative phenotype – Large circulating Sézary cells clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Staging of CTCL: LN Path stages clinicaloptions. com/oncology

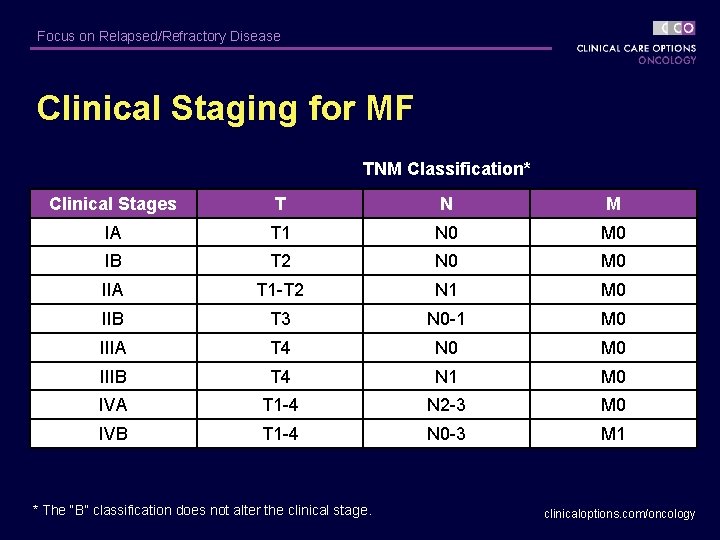
Focus on Relapsed/Refractory Disease Clinical Staging for MF TNM Classification* Clinical Stages T N M IA T 1 N 0 M 0 IB T 2 N 0 M 0 IIA T 1 -T 2 N 1 M 0 IIB T 3 N 0 -1 M 0 IIIA T 4 N 0 M 0 IIIB T 4 N 1 M 0 IVA T 1 -4 N 2 -3 M 0 IVB T 1 -4 N 0 -3 M 1 * The “B” classification does not alter the clinical stage. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL: Survival By Stage § Survival for CTCL patient varies according to stage § Little overlap in survival between stages – Stage IA: no median survival, similar to general population – Stage IB: 5 -year survival ~ 85% § Poor survival in advanced disease – Median survival for patients with stage IV CTCL: ~ 2. 5 years Kim, Y. H. et al. Arch Dermatol 2003; 139: 857 -866 clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease MF Histopathology § Surface antigen immunophenotyping – Classic antigenic profile: CD 4+, CD 8 -, CD 26 -, CD 45 RO+ – Variable presence or loss of pan T-cell markers CD 5 and/or CD 7 § Early-stage lesions characterized by – Infiltrating “reactive” CD 8+ T-lymphocytes – Cutaneous dendritic cells (lost upon disease progression) § T-cell gene rearrangement testing to confirm clonality clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease MF: Recommended Staging Studies § Routine clinical evaluations should include: – History and physical examination – Complete blood count with differential – Examination of blood smear for quantification of atypical lymphocytes (Sézary cells) – Comprehensive chemistry panel – Lactate dehydrogenase (LDH) – Lymph node biopsy if clinically apparent nodes present clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease MF: Other Staging Studies § Other studies performed in special circumstances or for clinical trials § Bone marrow aspirate/biopsy – Involvement in patients with advanced disease rarely changes treatment recommendation § Imaging Studies (CT scans) – Generally of modest utility and rarely positive in early T 1 or T 2 disease – Reserved for patients with Sézary syndrome or for quantifying disease stage in patients with tumor stage and palpable adenopathy clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Differential Diagnosis of MF § It is important to distinguish between MF and other T-cell disorders involving the skin – CD 30+ cutaneous large T-cell lymphoma – CD 30 - cutaneous large T-cell lymphoma – Peripheral T-cell lymphoma (unspecified) – Lymphomatoid papulosis – Subcutaneous panniculitis-like T-cell lymphoma clinicaloptions. com/oncology

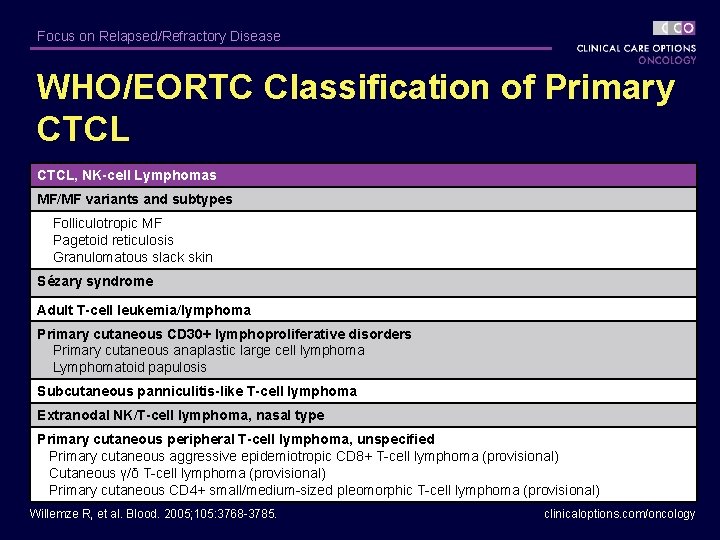
Focus on Relapsed/Refractory Disease WHO/EORTC Classification of Primary CTCL, NK-cell Lymphomas MF/MF variants and subtypes Folliculotropic MF Pagetoid reticulosis Granulomatous slack skin Sézary syndrome Adult T-cell leukemia/lymphoma Primary cutaneous CD 30+ lymphoproliferative disorders Primary cutaneous anaplastic large cell lymphoma Lymphomatoid papulosis Subcutaneous panniculitis-like T-cell lymphoma Extranodal NK/T-cell lymphoma, nasal type Primary cutaneous peripheral T-cell lymphoma, unspecified Primary cutaneous aggressive epidemiotropic CD 8+ T-cell lymphoma (provisional) Cutaneous γ/δ T-cell lymphoma (provisional) Primary cutaneous CD 4+ small/medium-sized pleomorphic T-cell lymphoma (provisional) Willemze R, et al. Blood. 2005; 105: 3768 -3785. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Pathogenesis of MF Skin homing T cells clinicaloptions. com/oncology

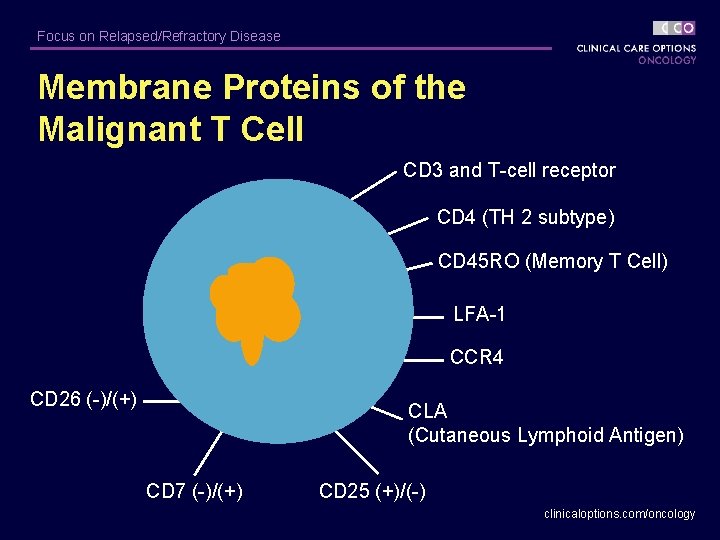
Focus on Relapsed/Refractory Disease Membrane Proteins of the Malignant T Cell CD 3 and T-cell receptor CD 4 (TH 2 subtype) CD 45 RO (Memory T Cell) LFA-1 CCR 4 CD 26 (-)/(+) CLA (Cutaneous Lymphoid Antigen) CD 7 (-)/(+) CD 25 (+)/(-) clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Pathogenesis of MF CCR 4 αEβ 7 Pautrier’s microabscess E-selectin Endothelial cell TCR CCL 22 CD 4 E-cadherin Langerhans’ cell MHC-II Epidermis Epidermotropism CLA CCR 4 T cell CCL 17 Dermis Capillary Extravasation Girardi M et al. N Eng J Med. 2004; 350: 1978 -1988. Copyright © 2004 Massachusetts Medical Society. All rights reserved. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL: Prognostic Groups § Low-risk group (most favorable): TNM stages IA, IB, IIA. – Survival: ~ 12 years § Intermediate risk group: TNM stages IIB, III, IVA with grade LN 3 lymph node histopathology – Survival: ~ 2 -3 years § High-risk group (least favorable): TNM stage IVB, IVA with grade 2 years LN 4 lymph node histopathology – Survival: ~ < 2 years Foss FM, Sausville EA. Hematol Oncol Clin North Am. 1995; 9: 1011 -1019. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL: Skin-Directed Therapy § Topical steroids § Topical nitrogen mustard (NM) – Mechlorethamine § Phototherapy – NBUVB – PUVA § Radiotherapy § Topical bexarotene gel – Total skin EBRT § Topical carmustine – Localized EBRT clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL: Systemic Therapy § Tretinoin capsules § Bexarotene capsules § Acitretin capsules § Methotrexate § Prednisone/chlorambucil § Extracorporeal photochemotherapy § Other biologic modifiers § Denileukin diftitox § Combination chemotherapy § Interferon clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL Treatment Algorithm Stage IA Stage IB, IIA Stage IIB Stage III Stage IVA, B Topical corticosteroids (Class I) Photopheresis ± IFN ± Bexarotene gel PUVA (± IFN or ± Retinoid) Electron Beam Bexarotene capsules Denileukin Diftitox Nitrogen Mustard UVB Photopheresis Chemotherapy or Allo. SCT clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL: Topical Therapies clinicaloptions. com/oncology

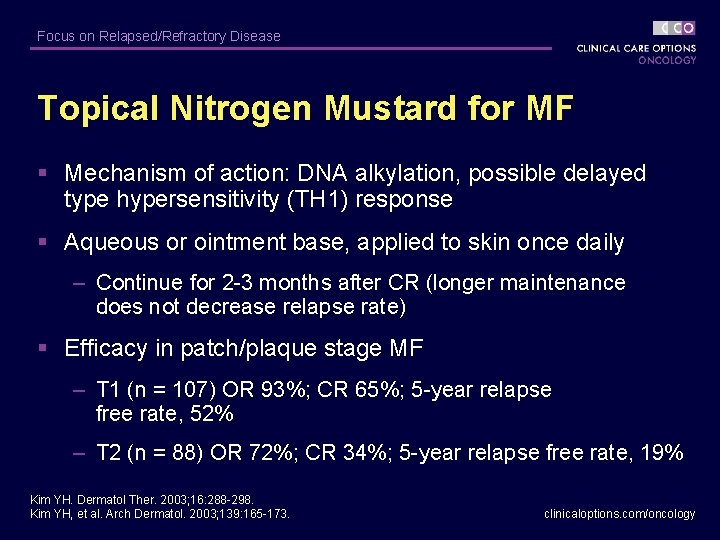
Focus on Relapsed/Refractory Disease Topical Nitrogen Mustard for MF § Mechanism of action: DNA alkylation, possible delayed type hypersensitivity (TH 1) response § Aqueous or ointment base, applied to skin once daily – Continue for 2 -3 months after CR (longer maintenance does not decrease relapse rate) § Efficacy in patch/plaque stage MF – T 1 (n = 107) OR 93%; CR 65%; 5 -year relapse free rate, 52% – T 2 (n = 88) OR 72%; CR 34%; 5 -year relapse free rate, 19% Kim YH. Dermatol Ther. 2003; 16: 288 -298. Kim YH, et al. Arch Dermatol. 2003; 139: 165 -173. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Topical Nitrogen Mustard for MF § Adverse effects – Irritant dermatitis < 25% (ointment) – Allergic contact dermatitis < 66% (aqueous), < 10% (ointment) – No systemic absorption – Possible slight increase in nonmelanoma skin cancers Kim YH. Dermatol Ther. 2003; 16: 288 -298. Kim YH, et al. Arch Dermatol. 2003; 139: 165 -173. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Topical Bexarotene for MF § Mechanism of action: Specific retinoid X receptor ligand that influences critical pathways for cell proliferation, differentiation, and apoptosis § Formulation: 1% gel, applied to affected skin, 1 -2 cm margins – Applied every other day first week, once daily second week, up to 4 times daily as tolerated § Adverse effects – Irritant “retinoid” dermatitis, 70% – Intervene with dose reduction, mildly potent topical steroids Zhang C, Duvic M. Dermatol Ther. 2003; 16: 322 -330. Kempf W, et al. Hematol Oncol Clin N Am. 2003; 17: 1405 -1419. clinicaloptions. com/oncology

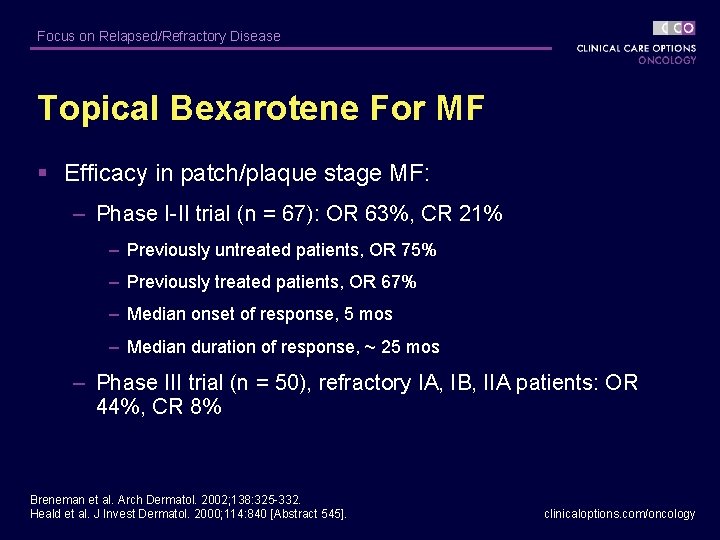
Focus on Relapsed/Refractory Disease Topical Bexarotene For MF § Efficacy in patch/plaque stage MF: – Phase I-II trial (n = 67): OR 63%, CR 21% – Previously untreated patients, OR 75% – Previously treated patients, OR 67% – Median onset of response, 5 mos – Median duration of response, ~ 25 mos – Phase III trial (n = 50), refractory IA, IB, IIA patients: OR 44%, CR 8% Breneman et al. Arch Dermatol. 2002; 138: 325 -332. Heald et al. J Invest Dermatol. 2000; 114: 840 [Abstract 545]. clinicaloptions. com/oncology

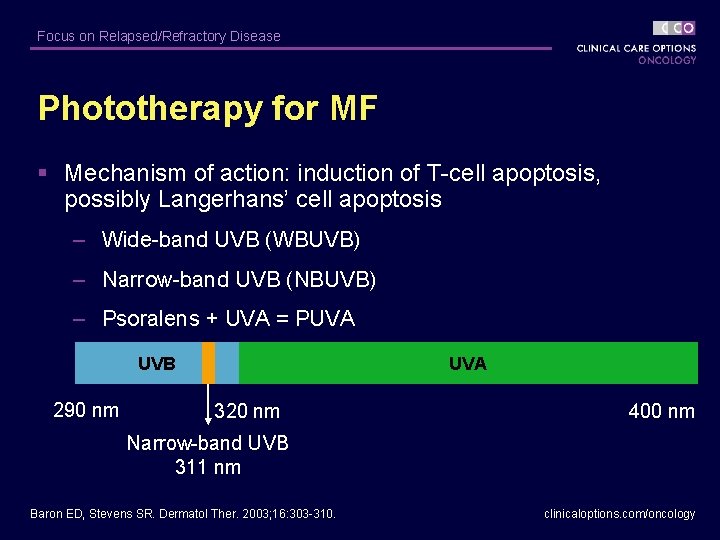
Focus on Relapsed/Refractory Disease Phototherapy for MF § Mechanism of action: induction of T-cell apoptosis, possibly Langerhans’ cell apoptosis – Wide-band UVB (WBUVB) – Narrow-band UVB (NBUVB) – Psoralens + UVA = PUVA UVB 290 nm UVA 320 nm 400 nm Narrow-band UVB 311 nm Baron ED, Stevens SR. Dermatol Ther. 2003; 16: 303 -310. clinicaloptions. com/oncology

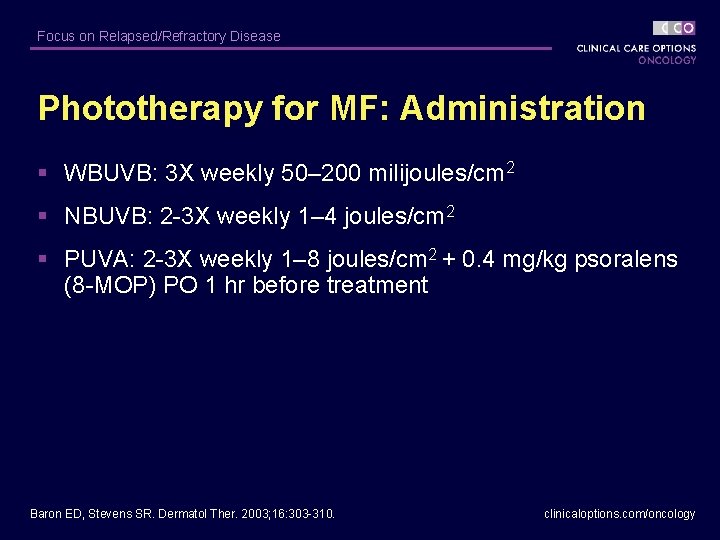
Focus on Relapsed/Refractory Disease Phototherapy for MF: Administration § WBUVB: 3 X weekly 50– 200 milijoules/cm 2 § NBUVB: 2 -3 X weekly 1– 4 joules/cm 2 § PUVA: 2 -3 X weekly 1– 8 joules/cm 2 + 0. 4 mg/kg psoralens (8 -MOP) PO 1 hr before treatment Baron ED, Stevens SR. Dermatol Ther. 2003; 16: 303 -310. clinicaloptions. com/oncology

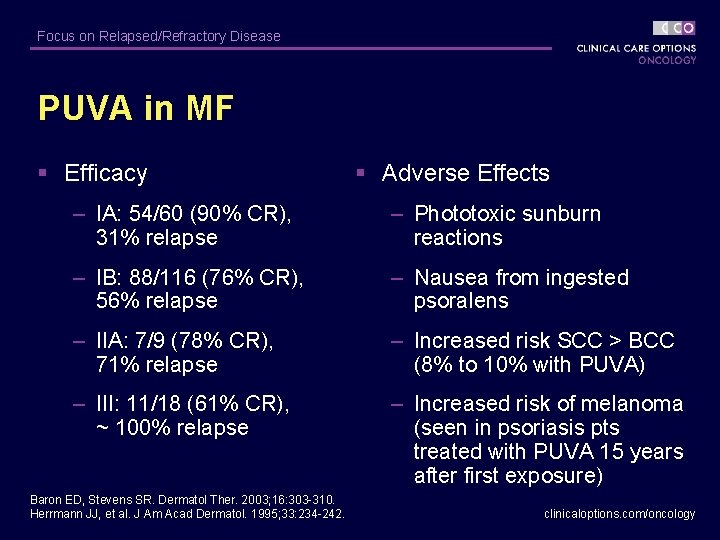
Focus on Relapsed/Refractory Disease PUVA in MF § Efficacy § Adverse Effects – IA: 54/60 (90% CR), 31% relapse – Phototoxic sunburn reactions – IB: 88/116 (76% CR), 56% relapse – Nausea from ingested psoralens – IIA: 7/9 (78% CR), 71% relapse – Increased risk SCC > BCC (8% to 10% with PUVA) – III: 11/18 (61% CR), ~ 100% relapse – Increased risk of melanoma (seen in psoriasis pts treated with PUVA 15 years after first exposure) Baron ED, Stevens SR. Dermatol Ther. 2003; 16: 303 -310. Herrmann JJ, et al. J Am Acad Dermatol. 1995; 33: 234 -242. clinicaloptions. com/oncology

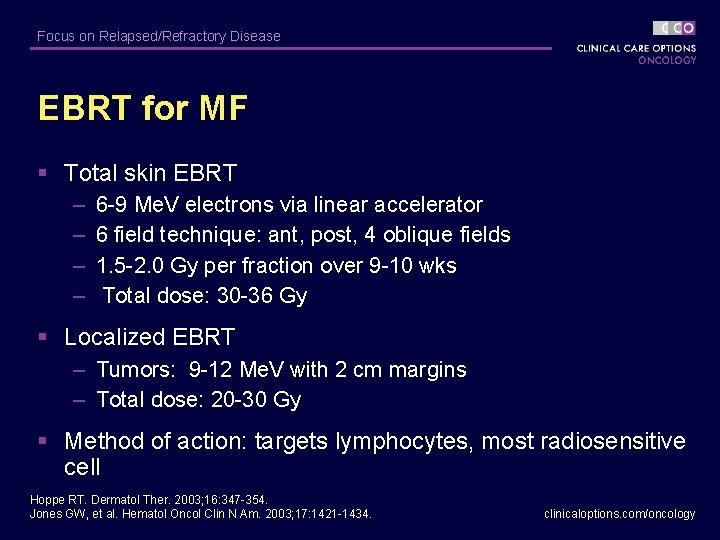
Focus on Relapsed/Refractory Disease EBRT for MF § Total skin EBRT – – 6 -9 Me. V electrons via linear accelerator 6 field technique: ant, post, 4 oblique fields 1. 5 -2. 0 Gy per fraction over 9 -10 wks Total dose: 30 -36 Gy § Localized EBRT – Tumors: 9 -12 Me. V with 2 cm margins – Total dose: 20 -30 Gy § Method of action: targets lymphocytes, most radiosensitive cell Hoppe RT. Dermatol Ther. 2003; 16: 347 -354. Jones GW, et al. Hematol Oncol Clin N Am. 2003; 17: 1421 -1434. clinicaloptions. com/oncology

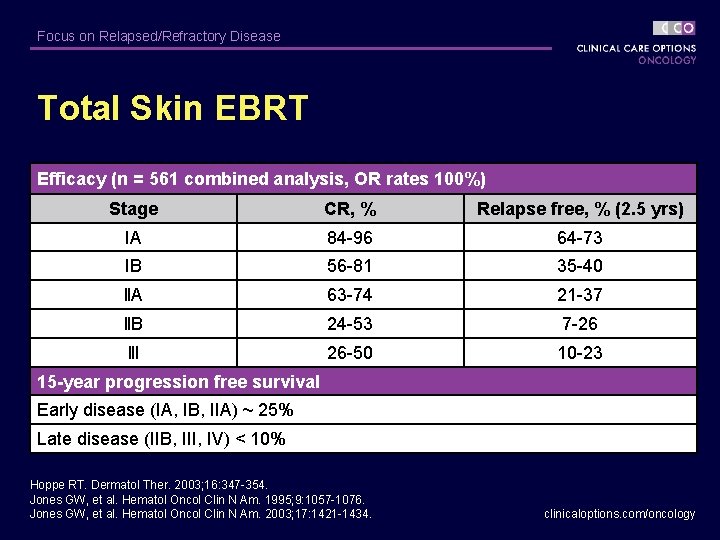
Focus on Relapsed/Refractory Disease Total Skin EBRT Efficacy (n = 561 combined analysis, OR rates 100%) Stage CR, % Relapse free, % (2. 5 yrs) IA 84 -96 64 -73 IB 56 -81 35 -40 ll. A 63 -74 21 -37 ll. B 24 -53 7 -26 lll 26 -50 10 -23 15 -year progression free survival Early disease (IA, IB, IIA) ~ 25% Late disease (IIB, III, IV) < 10% Hoppe RT. Dermatol Ther. 2003; 16: 347 -354. Jones GW, et al. Hematol Oncol Clin N Am. 1995; 9: 1057 -1076. Jones GW, et al. Hematol Oncol Clin N Am. 2003; 17: 1421 -1434. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Total Skin EBRT § Adverse effects – Acute skin effects: burning erythema, edema – Chronic skin effects: xerosis, superficial atrophy, telangiectasia, dyspigmentation – Alopecia, loss of nails (usually regrow) – Heat intolerance due to the suppression of sweat gland production (usual duration, 6 -12 mos) – Increased SCC and BCC – Otherapies may play a role Hoppe RT. Dermatol Ther. 2003; 16: 347 -354 Jones GW, et al. Hematol Oncol Clin N Am. 2003; 17: 1421 -1434. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease CTCL: Systemic Therapies clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Oral Retinoids for MF/Sézary Syndrome § Specific retinoid receptor ligands that influence critical pathways for cell proliferation, differentiation, and apoptosis Retinoid Daily Dose Acitretin 25 -50 mg/d Bexarotene* 300 mg/m 2 BSA/day Isotretinoin 1 mg/kg/d * FDA-approved for CTCL in December 1999 Zhang C, Duvic M. Dermatol Ther. 2003; 16: 322 -330. Kempf W, et al. Hematol Oncol Clin N Am. 2003; 17: 1405 -1419. clinicaloptions. com/oncology

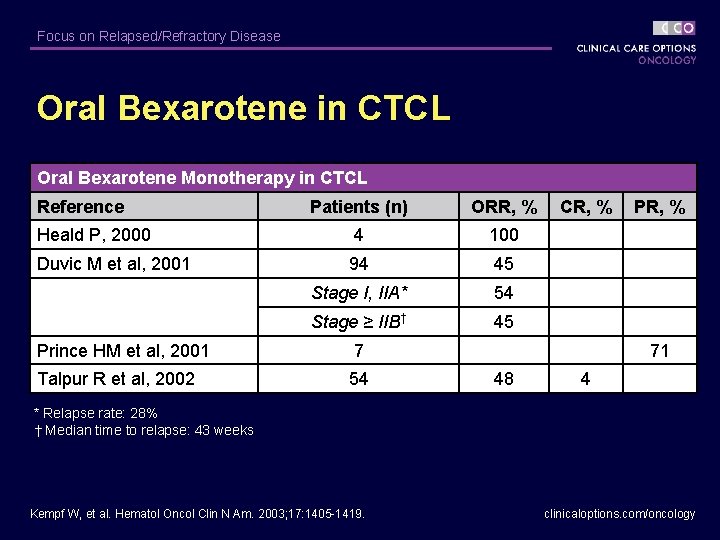
Focus on Relapsed/Refractory Disease Oral Bexarotene in CTCL Oral Bexarotene Monotherapy in CTCL Reference Patients (n) ORR, % CR, % PR, % Heald P, 2000 4 100 Duvic M et al, 2001 94 45 Stage I, IIA* 54 Stage ≥ IIB† 45 Prince HM et al, 2001 7 71 Talpur R et al, 2002 54 48 4 * Relapse rate: 28% † Median time to relapse: 43 weeks Kempf W, et al. Hematol Oncol Clin N Am. 2003; 17: 1405 -1419. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Oral Bexarotene in CTCL (continued) Baseline Week 12 Week 28 clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Oral Bexarotene for MF/Sézary Syndrome § Adverse effects: hyperlipidemia (> 80%): usually requires treatment with lipid lowering agent – Triglyceride level of 700 -900 mg/d. L in patients on treatment warrants therapy interruption for 5 -7 days – Addition of lipid-lowering agent; resume therapy at half initial dose, monitor triglycerides once weekly thereafter – Initiation of lipid-lowering agent recommended 1 week before starting oral bexarotene – Diet: reduction of fat/sugar intake – Initiating with low-dose bexarotene feasible in some patients (eg, those with diabetes) Zhang C, Duvic M. Dermatol Ther. 2003; 16: 322 -330. Kempf W, et al. Hematol Oncol Clin N Am. 2003; 17: 1405 -1419. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Oral Bexarotene for MF/Sézary Syndrome § Other adverse effects: – Leukopenia (11%) – Central hypothyroidism (30% to > 70%): may require thyroid supplementation – Gemfibrozil contraindicated Zhang C, Duvic M. Dermatol Ther. 2003; 16: 322 -330. Kempf W, et al. Hematol Oncol Clin N Am. 2003; 17: 1405 -1419. clinicaloptions. com/oncology

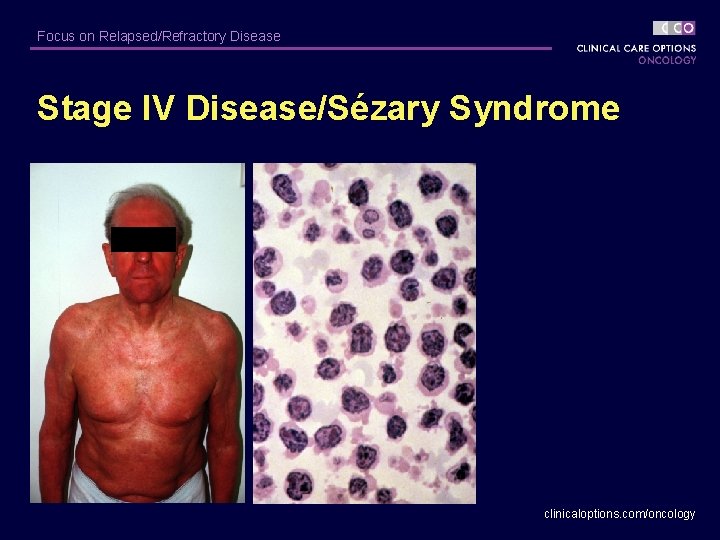
Focus on Relapsed/Refractory Disease Stage IV Disease/Sézary Syndrome clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Sézary Syndrome § Characterized by erythroderma, circulating Sézary cells, adenopathy – Secondary characteristics: alopecia, onychodystrophy, palmar/plantar keratoderma § EORTC suggests: TCRR (+) peripheral blood, CD 4/CD 8 > 10 for diagnosis of Sézary syndrome. § Prognosis poor: median survival, < 20 -36 mos clinicaloptions. com/oncology

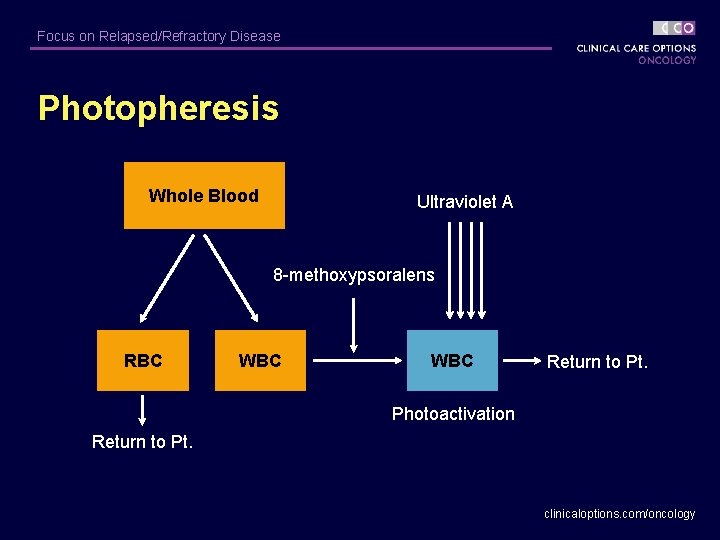
Focus on Relapsed/Refractory Disease Photopheresis Whole Blood Ultraviolet A 8 -methoxypsoralens RBC WBC Return to Pt. Photoactivation Return to Pt. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Photopheresis for MF/Sézary Syndrome § CTCL protocol: one 3 -hour treatment on 2 consecutive days, every 4 weeks § Proposed mechanism of action – Induces apoptosis of lymphocytes – Converts monocytes to immature dendritic cells – Dendritic cells engulf lymphocytes and present tumor antigen to cytotoxic T cells Zic JA. Dermatol Ther. 2003; 16: 337 -346. clinicaloptions. com/oncology

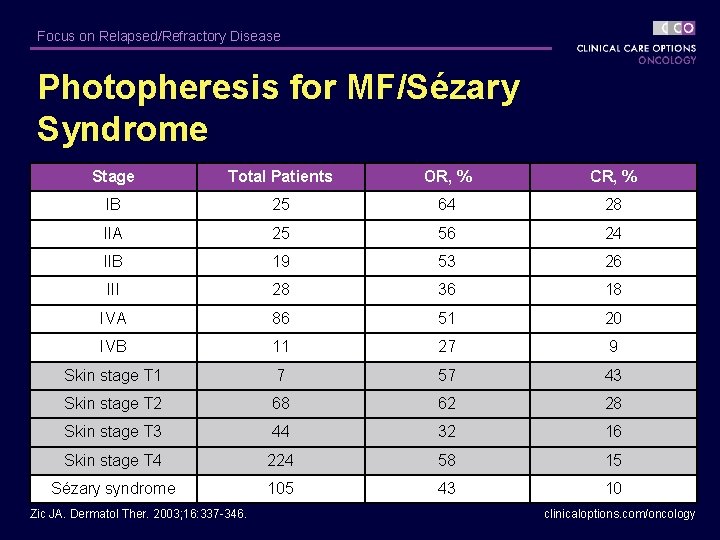
Focus on Relapsed/Refractory Disease Photopheresis for MF/Sézary Syndrome Stage Total Patients OR, % CR, % IB 25 64 28 IIA 25 56 24 IIB 19 53 26 III 28 36 18 IVA 86 51 20 IVB 11 27 9 Skin stage T 1 7 57 43 Skin stage T 2 68 62 28 Skin stage T 3 44 32 16 Skin stage T 4 224 58 15 Sézary syndrome 105 43 10 Zic JA. Dermatol Ther. 2003; 16: 337 -346. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Denileukin Diftitox for CTCL § Diphtheria toxin fragments A and B linked to interleukin-2 – Approximately 50% CTCL tumor cells express interleukin-2 receptor § Pivotal study (N = 71) in pretreated patients, most with stage IIB-IVB disease (63%) – 30% overall response – Median duration of response, 4 mos Olsen E, et al. J Clin Oncol. 2001; 19: 376 -388. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Denileukin Diftitox for CTCL: Adverse Events § Acute hypersensitivity reactions (69%): acute; hypotension, SOB, rash, chest pain – Pretreatment with steroids may improve tolerability, maintain response § Vascular leak syndrome (27%): delayed; hypotension, edema, hypoalbuminemia § Infections (48%) § Lymphopenia (34%) Olsen E, et al. J Clin Oncol. 2001; 19: 376 -388. Foss FM et al. Clin Lymphoma. 2001; 4: 298 -302. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Chemotherapy for CTCL § Modest response, reserved for relapsed or refractory disease § Duration of response < 6 mos § Some agents show notable activity: – Deoxycoformycin – Gemcitabine: phase II trial (N = 32) untreated CTCL, 7 (22%) CR, 17 (53%) PR – Pegylated liposomal doxorubicin: retrospective analysis (N = 34) CTCL patients, 15 CR (DFS 13. 3 mo), 15 PR Kuzel TM. Dermatol Ther. 2003; 16: 355 -361. Pichardo DA, et al. Leuk Lymphoma. 2004; 45: 1755 -1765. Marchi E, et al. Cancer. 2005; 104: 2437 -2441. Wollina U, et al. Cancer. 2003; 98: 993 -1001. clinicaloptions. com/oncology

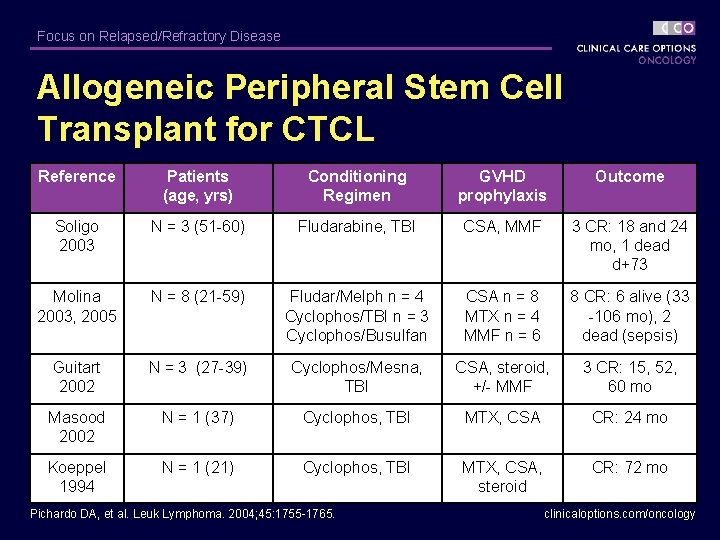
Focus on Relapsed/Refractory Disease Allogeneic Peripheral Stem Cell Transplant for CTCL Reference Patients (age, yrs) Conditioning Regimen GVHD prophylaxis Outcome Soligo 2003 N = 3 (51 -60) Fludarabine, TBI CSA, MMF 3 CR: 18 and 24 mo, 1 dead d+73 Molina 2003, 2005 N = 8 (21 -59) Fludar/Melph n = 4 Cyclophos/TBI n = 3 Cyclophos/Busulfan CSA n = 8 MTX n = 4 MMF n = 6 8 CR: 6 alive (33 -106 mo), 2 dead (sepsis) Guitart 2002 N = 3 (27 -39) Cyclophos/Mesna, TBI CSA, steroid, +/- MMF 3 CR: 15, 52, 60 mo Masood 2002 N = 1 (37) Cyclophos, TBI MTX, CSA CR: 24 mo Koeppel 1994 N = 1 (21) Cyclophos, TBI MTX, CSA, steroid CR: 72 mo Pichardo DA, et al. Leuk Lymphoma. 2004; 45: 1755 -1765. clinicaloptions. com/oncology

Focus on Relapsed/Refractory Disease Summary of Therapies for CTCL § Skin-directed therapies: highly effective in the early stages of MF – Relapses common § The challenge: develop systemic targeted therapies with minimal adverse effects capable of inducing meaningful remissions § Current therapeutic goal: do no harm, prevent disease progression § Comparison trials needed to prioritize systemic therapy clinicaloptions. com/oncology
 Primary cutaneous gamma/delta t-cell lymphoma
Primary cutaneous gamma/delta t-cell lymphoma Lymphoma
Lymphoma Focus on form vs focus on forms
Focus on form vs focus on forms Porter's competitive strategies
Porter's competitive strategies Differentiation business level strategy
Differentiation business level strategy Actor focus vs object focus
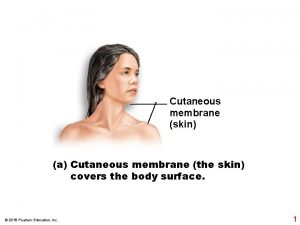
Actor focus vs object focus Cutaneous membrane
Cutaneous membrane Five layer of skin
Five layer of skin Medial plantar nerve
Medial plantar nerve Palmar cutaneous branch of median nerve
Palmar cutaneous branch of median nerve Femeral vein
Femeral vein Cutaneous reflex
Cutaneous reflex Cutaneous membrane
Cutaneous membrane Entamoeba
Entamoeba Neuron order
Neuron order Sacral plexus
Sacral plexus Triple response and cutaneous circulation
Triple response and cutaneous circulation Cutaneous innervation of scalp
Cutaneous innervation of scalp Lateral femoral cutaneous nerve
Lateral femoral cutaneous nerve Median nerve innervation
Median nerve innervation Scalp innervation
Scalp innervation Harold varmus
Harold varmus Cutaneous reflex
Cutaneous reflex Palmar cutaneous branch of median nerve
Palmar cutaneous branch of median nerve Cutaneous innervation of arm
Cutaneous innervation of arm Free edge
Free edge Lateral plantar nerve
Lateral plantar nerve Cutaneous receptors
Cutaneous receptors Polychromasia
Polychromasia Ventral vs dorsal
Ventral vs dorsal Musculocutaneous nerve supply
Musculocutaneous nerve supply Cutaneous membrane definition
Cutaneous membrane definition What are the four primary tissue types
What are the four primary tissue types Cutaneous mycoses
Cutaneous mycoses Cutaneous membrane
Cutaneous membrane Ilioinguinal nerve
Ilioinguinal nerve Double life animal
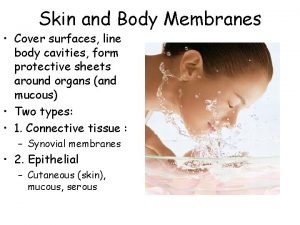
Double life animal Types of body membranes
Types of body membranes Maintenance rituximab mantle cell lymphoma
Maintenance rituximab mantle cell lymphoma Classification of hodgkin lymphoma
Classification of hodgkin lymphoma Hiv family name
Hiv family name Hodgkin's lymphoma classification
Hodgkin's lymphoma classification Gadolinium mrt
Gadolinium mrt Szóbajön
Szóbajön Lymphoma vs leukemia
Lymphoma vs leukemia Lymphoma classification ppt
Lymphoma classification ppt Hepatosplenic t-cell lymphoma
Hepatosplenic t-cell lymphoma Neoplasia
Neoplasia Indolent non-hodgkin lymphoma quizlet
Indolent non-hodgkin lymphoma quizlet Nonhodgkins lymphoma
Nonhodgkins lymphoma Non-hodgkin lymphoma
Non-hodgkin lymphoma Lymphoma alcohol
Lymphoma alcohol Chylothorax
Chylothorax Smoldering lymphoma
Smoldering lymphoma