TRANSPORT OF IONS IN SOLUTION Conductivity of electrolyte











- Slides: 12

TRANSPORT OF IONS IN SOLUTION § Conductivity of electrolyte solutions § Strong and weak electrolyte Jaslin Ikhsan, Ph. D. Chemistry Ed. Department State University of Yogyakarta

• Mahasiswa dapat menjelaskan pengertian konduktansi dan konduktivitas • Mahasiswa dapat menghitung konduktivitas molar larutan • Mahasiswa dapat menjelaskan hukum pengenceran Ostwald • Mahasiswa dapat menentukan p. Ka dengan menggunakan hasil pengukuran konduktivitas

Conductivity of Electrolyte Solution § Ions in solution can be set in motion by applying a potential difference between two electrodes. § The conductance (G) of a solution is defined as the inverse of the resistance (R): § For parallel plate electrodes with area A, it follows: Where, Κ: the conductivity, L : the distance separating the plates Units: G → S (siemens) R→Ω κ → S m-1

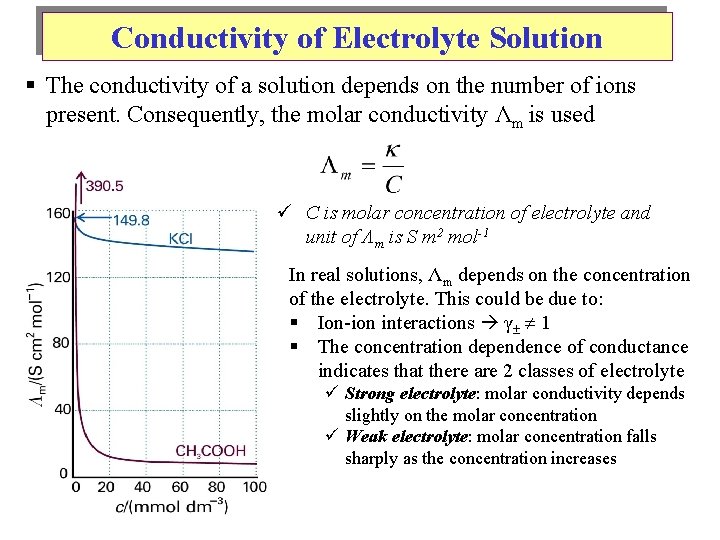
Conductivity of Electrolyte Solution § The conductivity of a solution depends on the number of ions present. Consequently, the molar conductivity Λm is used ü C is molar concentration of electrolyte and unit of Λm is S m 2 mol-1 In real solutions, Λm depends on the concentration of the electrolyte. This could be due to: § Ion-ion interactions γ 1 § The concentration dependence of conductance indicates that there are 2 classes of electrolyte ü Strong electrolyte: molar conductivity depends slightly on the molar concentration ü Weak electrolyte: molar concentration falls sharply as the concentration increases

Conductivity of Electrolyte Solution In real solutions, Λm depends on the concentration of the electrolyte. This could be due to: 1. Ion-ion interactions γ 1 2. Incomplete dissociation of electrolyte strong electrolyte, weak dependence of Λm on C weak electrolyte, strong dependence of Λm on C

Strong Electrolyte § Fully ionized in solution § Kohlrausch’s law üΛ 0 m is the limiting molar conductivity ü K is a constant which typically depends on the stoichiometry of the electrolyte § C 1/2 arises from ion-ion interactions as estimated by the Debye-Hückel theory.

Strong Electrolyte § Law of the independent migration of ions: limiting molar conductivity can be expressed as a sum of ions contribution ü ions migrate independently in the zero concentration limit

Weak Electrolyte § Not fully ionized in solution

Weak Electrolyte § The molar Conductivity (at higher concentrations) can be expressed as: § At infinite dilution, the weak acid is fully dissociated (α = 100%) § It can be proven by the Ostwald dilution law which allows estimating limiting molar conductance:

Weak Electrolyte § The limiting molar conductance: Hukum Pengenceran Ostwald Graph to determine the limiting value of the molar conductivity of a solution by extrapolation to zero concentration

Diskusi: 1. Konduktivitas molar larutan elektrolit pada 250 C adalah 135, 5 S cm 2 mol-1 dan konsentrasinya adalah 5, 35 x 10 -2 M. Hitunglah konduktivitas larutan! (20) 2. Sel konduktivitas mempunyai elektrode bidang yang sejajar, masing-masing luasnya 2, 2 cm x 2, 2 cm, dan terpisah sejauh 2, 75 cm. Jika sel diisi dengan larutan elektrolit, tahanannya adalah 351 ohm. Berapakah konduktivitas larutan? (25) 3. Pada 250 C konduktivitas larutan elektrolit kuat dalam air adalah 109, 9 S cm 2 mol-1 untuk konsentrasi 6, 2 x 10 -3 M dan 106, 1 S cm 2 mol-1 untuk konsentrasi 1, 50 x 10 -2 M. Berapakah konduktivitas molar pembatas elektrolit tersebut? (30) 4. Konduktivitas molar 0, 1000 M KCl (aq) adalah 129 S cm 2 mol-1 dan tahanan terukur dalam sel konduktivitas adalah 28, 44 ohm. Tahanan itu besarnya 28, 50 ohm jika sel yang sama berisi 0, 1000 M NH 4 Cl (aq). Hitunglah konduktivitas molar NH 4 Cl (aq) pada konsentrasi ini! (25)

Thank You
 What do the roman numerals in a cation's name indicate?
What do the roman numerals in a cation's name indicate? Electrolyte replacement therapy
Electrolyte replacement therapy Refeeding syndrome electrolyte abnormalities
Refeeding syndrome electrolyte abnormalities Blood chemistry
Blood chemistry Water electrolyte imbalance
Water electrolyte imbalance Principal cells
Principal cells Hyperkalemia signs and symptoms
Hyperkalemia signs and symptoms Fluid and electrolyte balance ppt
Fluid and electrolyte balance ppt Solubility curve
Solubility curve Chapter 26 fluid electrolyte and acid-base balance
Chapter 26 fluid electrolyte and acid-base balance Larox dm dual media electrolyte filter
Larox dm dual media electrolyte filter What is electrolyte
What is electrolyte Hf electrolyte
Hf electrolyte