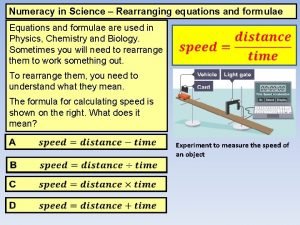
Topic 1 3 Formulae equations and amounts of







- Slides: 33

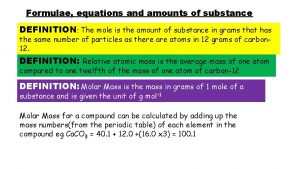
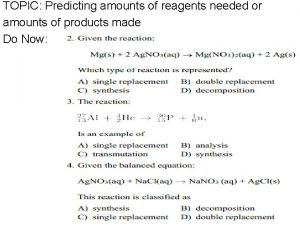
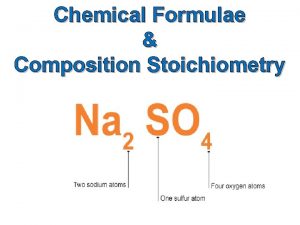
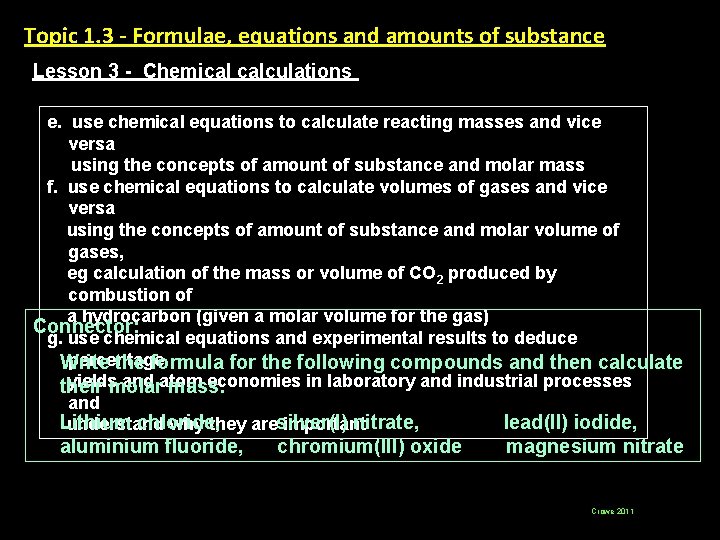
Topic 1. 3 - Formulae, equations and amounts of substance Lesson 3 - Chemical calculations e. use chemical equations to calculate reacting masses and vice versa using the concepts of amount of substance and molar mass f. use chemical equations to calculate volumes of gases and vice versa using the concepts of amount of substance and molar volume of gases, eg calculation of the mass or volume of CO 2 produced by combustion of a hydrocarbon (given a molar volume for the gas) Connector: g. use chemical equations and experimental results to deduce percentage Write the formula for the following compounds and then calculate yieldsmolar and atom economies in laboratory and industrial processes their mass. and Lithium chloride, nitrate, lead(II) iodide, understand why they aresilver(I) important aluminium fluoride, chromium(III) oxide magnesium nitrate Crowe 2011

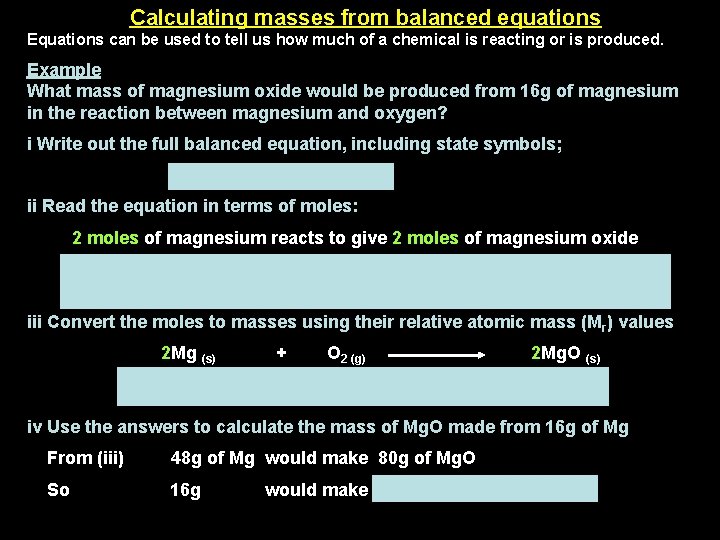
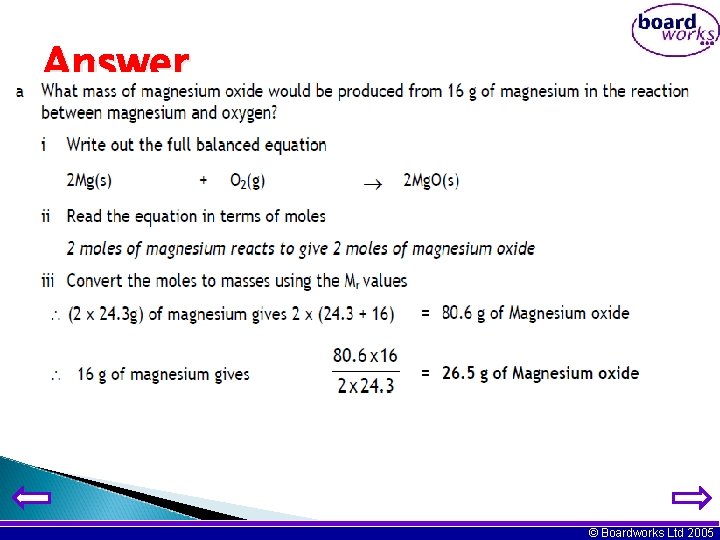
Calculating masses from balanced equations Equations can be used to tell us how much of a chemical is reacting or is produced. Example What mass of magnesium oxide would be produced from 16 g of magnesium in the reaction between magnesium and oxygen? i Write out the full balanced equation, including state symbols; 2 Mg (s) + O 2 (g) → 2 Mg. O (s) ii Read the equation in terms of moles: 2 moles of magnesium reacts to give 2 moles of magnesium oxide Note: The numbers written in front of the substances in an equation represent moles, where 1 mole = formula weight in grams. iii Convert the moles to masses using their relative atomic mass (Mr) values 2 Mg (s) (2 x 24 g) = 48 g + O 2 (g) 2 Mg. O (s) 2 x (24 + 16) = 80 g iv Use the answers to calculate the mass of Mg. O made from 16 g of Mg From (iii) 48 g of Mg would make 80 g of Mg. O So 16 g would make (80 x 16) / 48 = 26. 7 g

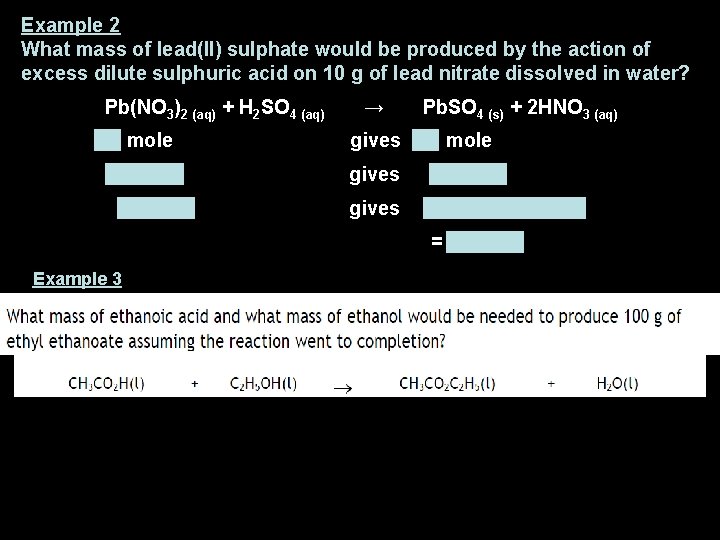
Example 2 What mass of lead(II) sulphate would be produced by the action of excess dilute sulphuric acid on 10 g of lead nitrate dissolved in water? Pb(NO 3)2 (aq) + H 2 SO 4 (aq) → Pb. SO 4 (s) + 2 HNO 3 (aq) 1 mole gives 1 mole 331. 2 g gives 303. 2 g 10 g gives ( 303. 2 x 10) / 331. 2 = 9. 15 g Example 3

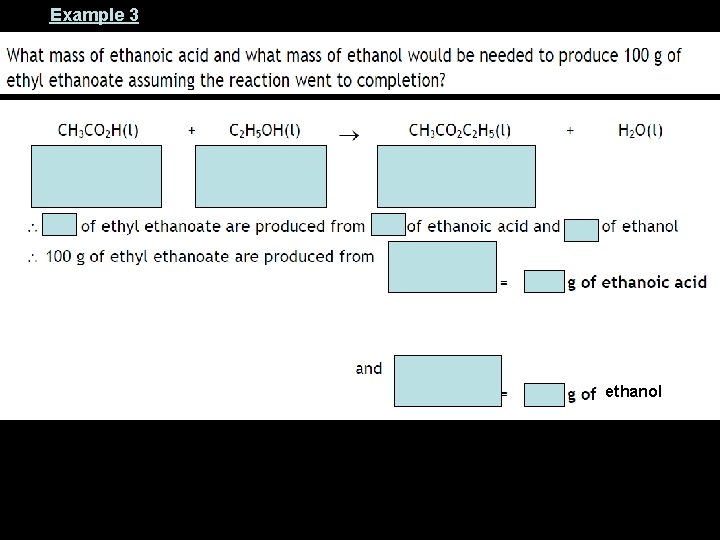
Example 3 ethanol




Atom economy • The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. • Inefficient, wasteful processes have low atom economies. • Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. • The atom economy of a reaction can be calculated: Note that, because the total mass of products equals the total mass of reactants, you can put that into the bottom of the fraction in the calculation like this:



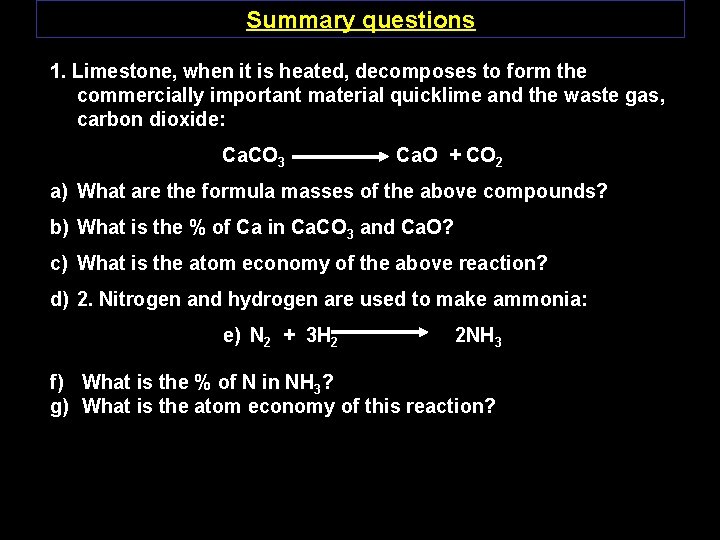
Summary questions 1. Limestone, when it is heated, decomposes to form the commercially important material quicklime and the waste gas, carbon dioxide: Ca. CO 3 Ca. O + CO 2 a) What are the formula masses of the above compounds? b) What is the % of Ca in Ca. CO 3 and Ca. O? c) What is the atom economy of the above reaction? d) 2. Nitrogen and hydrogen are used to make ammonia: e) N 2 + 3 H 2 2 NH 3 f) What is the % of N in NH 3? g) What is the atom economy of this reaction?

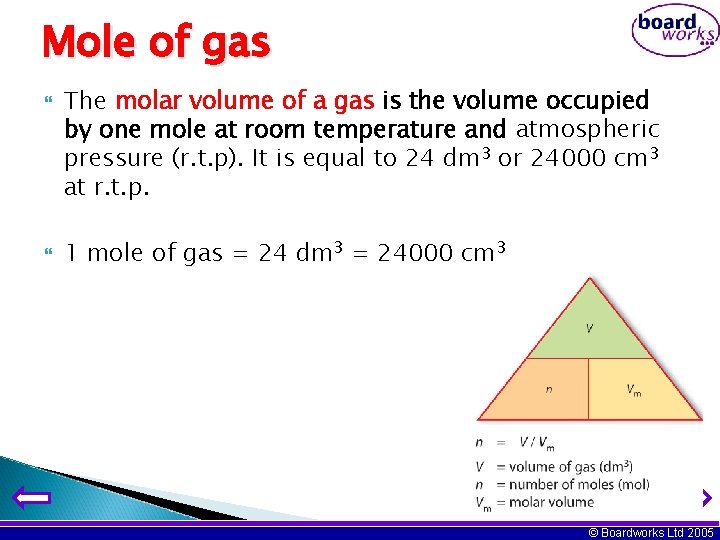
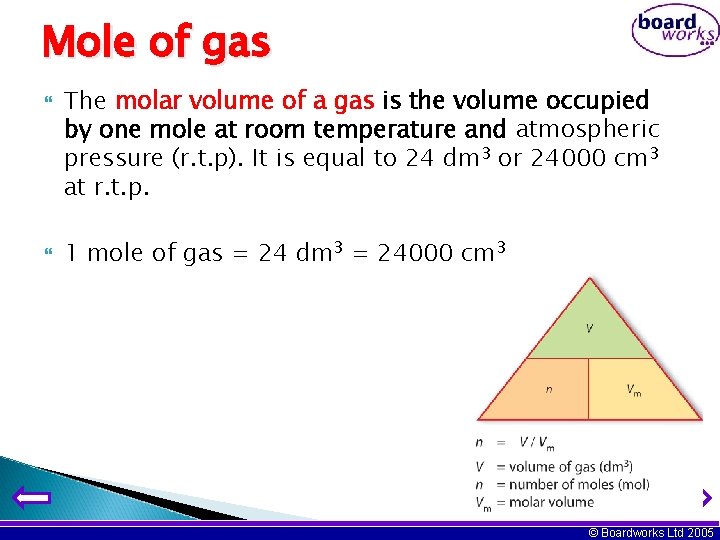
Mole of gas The molar volume of a gas is the volume occupied by one mole at room temperature and atmospheric pressure (r. t. p). It is equal to 24 dm 3 or 24000 cm 3 at r. t. p. 1 mole of gas = 24 dm 3 = 24000 cm 3 © Boardworks Ltd 2005

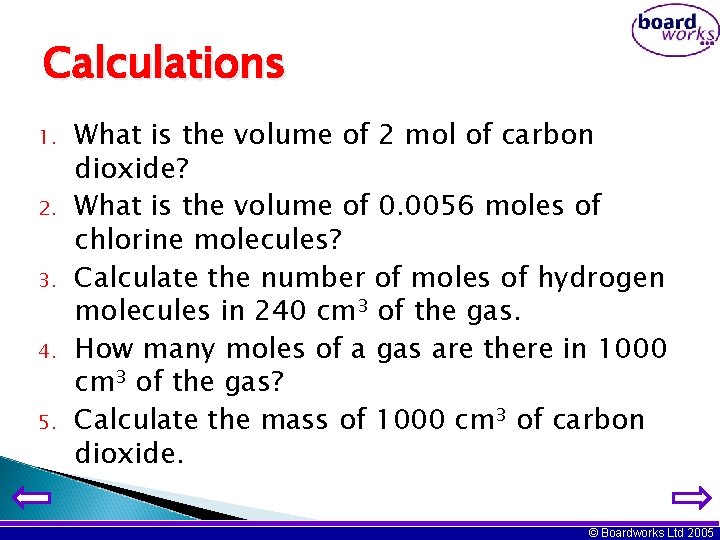
Calculations 1. 2. 3. 4. 5. What is the volume of 2 mol of carbon dioxide? What is the volume of 0. 0056 moles of chlorine molecules? Calculate the number of moles of hydrogen molecules in 240 cm 3 of the gas. How many moles of a gas are there in 1000 cm 3 of the gas? Calculate the mass of 1000 cm 3 of carbon dioxide. © Boardworks Ltd 2005

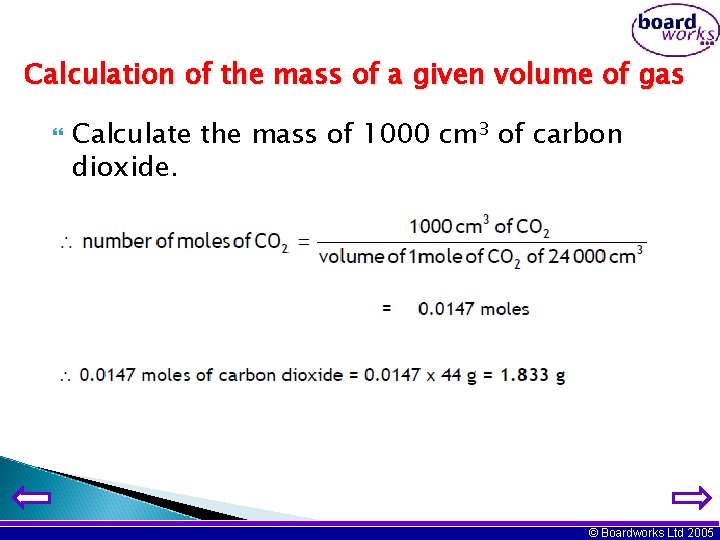
Calculation of the mass of a given volume of gas Calculate the mass of 1000 cm 3 of carbon dioxide. © Boardworks Ltd 2005

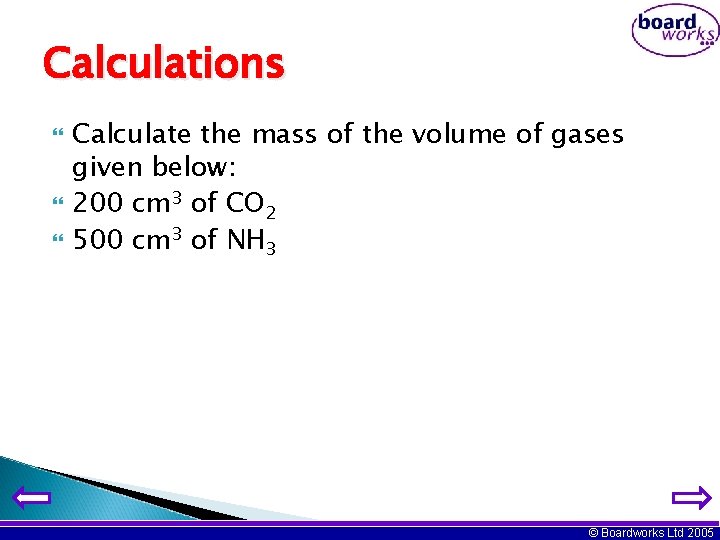
Calculations Calculate the mass of the volume of gases given below: 200 cm 3 of CO 2 500 cm 3 of NH 3 © Boardworks Ltd 2005

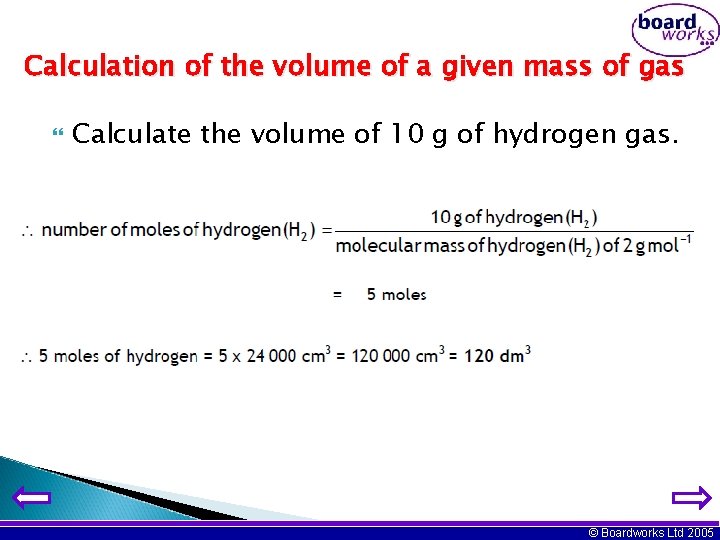
Calculation of the volume of a given mass of gas Calculate the volume of 10 g of hydrogen gas. © Boardworks Ltd 2005

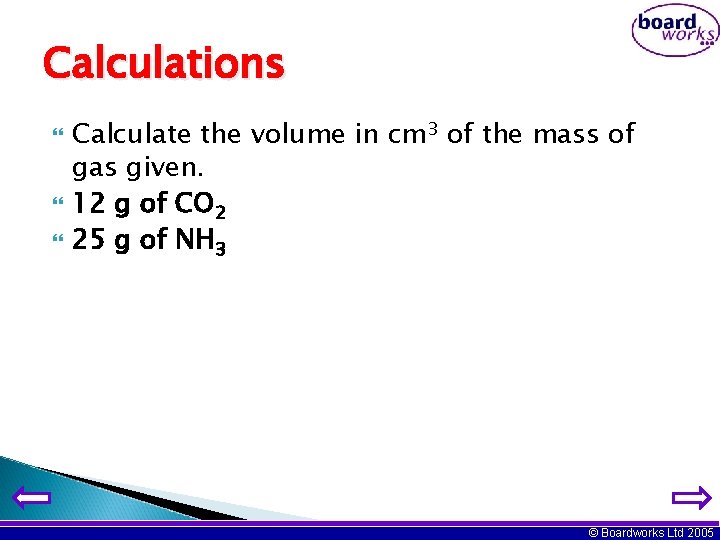
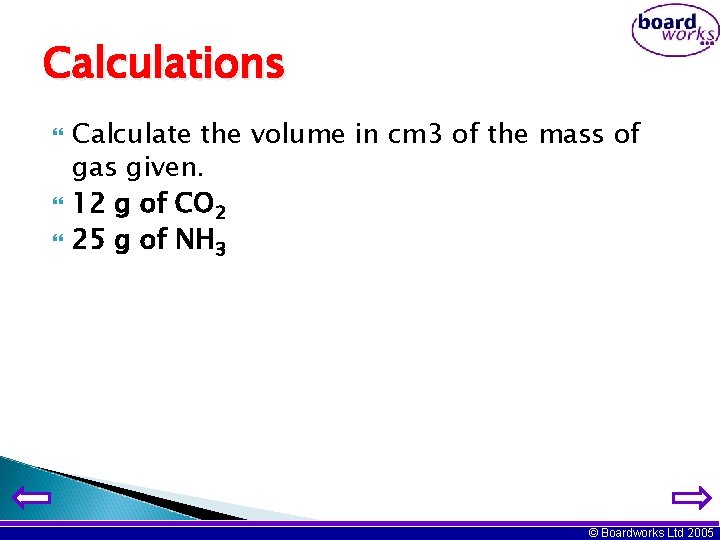
Calculations Calculate the volume in cm 3 of the mass of gas given. 12 g of CO 2 25 g of NH 3 © Boardworks Ltd 2005

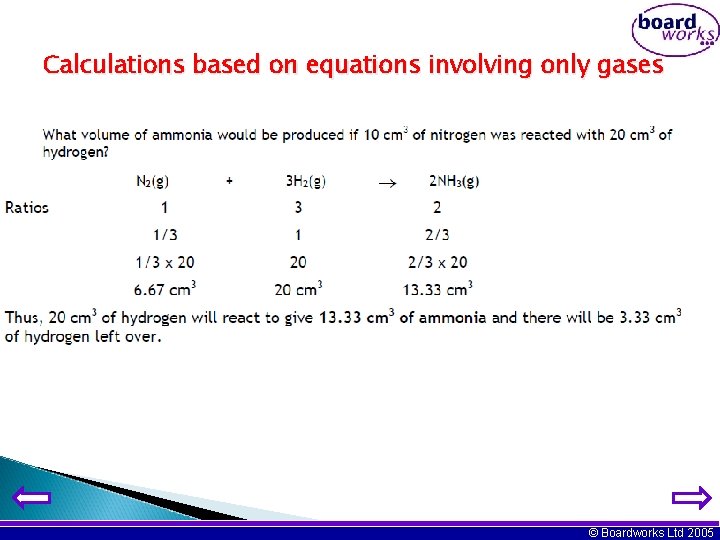
Calculations based on equations involving only gases © Boardworks Ltd 2005

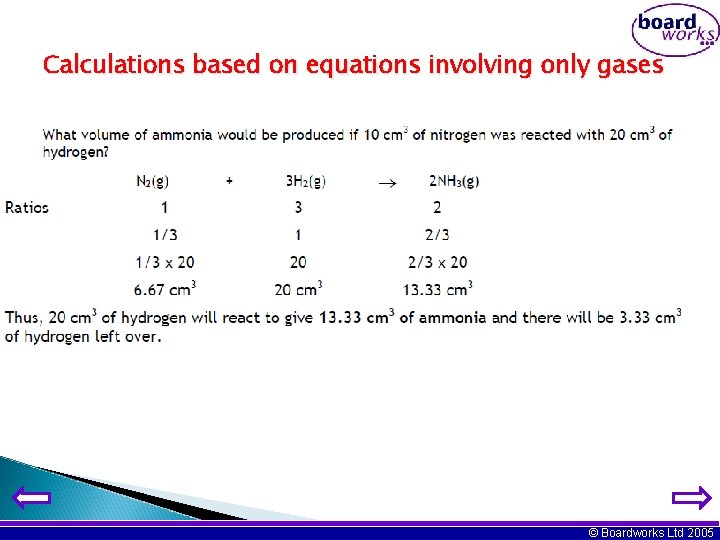
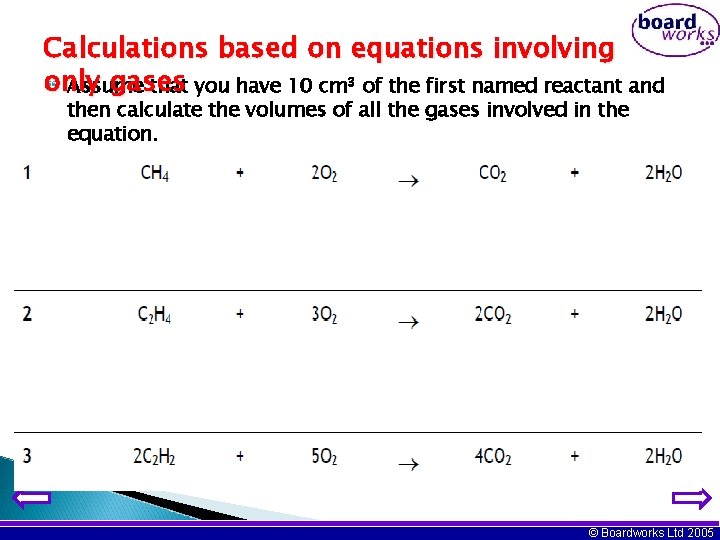
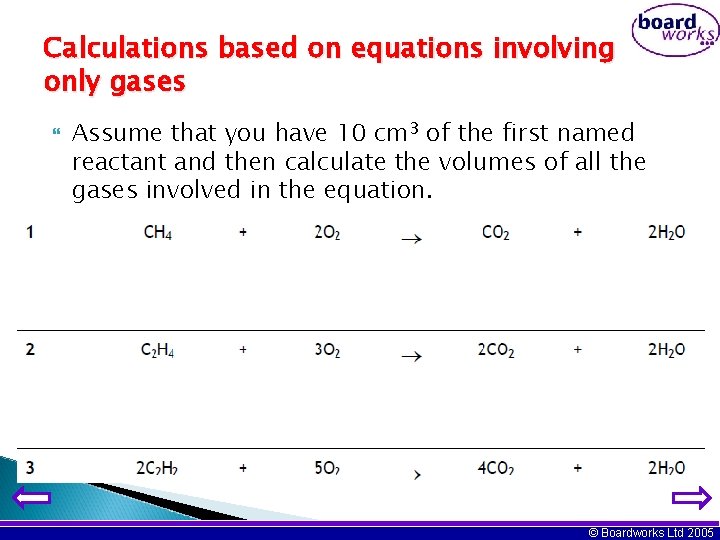
Calculations based on equations involving only gases Assume that you have 10 cm 3 of the first named reactant and then calculate the volumes of all the gases involved in the equation. © Boardworks Ltd 2005

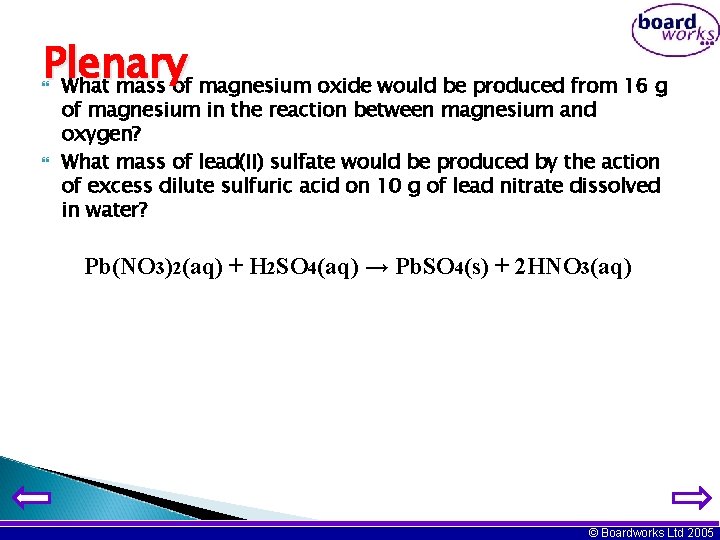
Plenary What mass of magnesium oxide would be produced from 16 g of magnesium in the reaction between magnesium and oxygen? What mass of lead(II) sulfate would be produced by the action of excess dilute sulfuric acid on 10 g of lead nitrate dissolved in water? Pb(NO 3)2(aq) + H 2 SO 4(aq) → Pb. SO 4(s) + 2 HNO 3(aq) © Boardworks Ltd 2005

Answer © Boardworks Ltd 2005

Answer © Boardworks Ltd 2005

Mole Calculate the mass of reactant and product Calculate the volume and mass of gas © Boardworks Ltd 2005

Mole of gas The molar volume of a gas is the volume occupied by one mole at room temperature and atmospheric pressure (r. t. p). It is equal to 24 dm 3 or 24000 cm 3 at r. t. p. 1 mole of gas = 24 dm 3 = 24000 cm 3 © Boardworks Ltd 2005

Avogadro’s law At the same temperature and pressure, equal volumes of gases will contain the same number of gas particles, and so the same number of moles. © Boardworks Ltd 2005

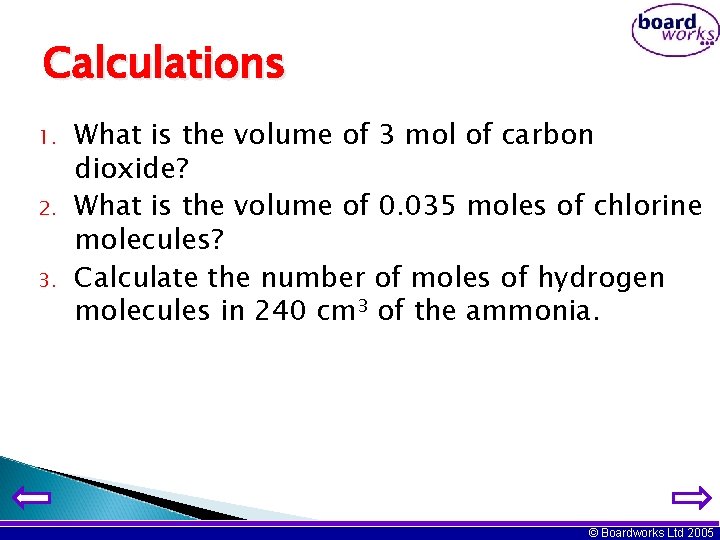
Calculations 1. 2. 3. What is the volume of 3 mol of carbon dioxide? What is the volume of 0. 035 moles of chlorine molecules? Calculate the number of moles of hydrogen molecules in 240 cm 3 of the ammonia. © Boardworks Ltd 2005

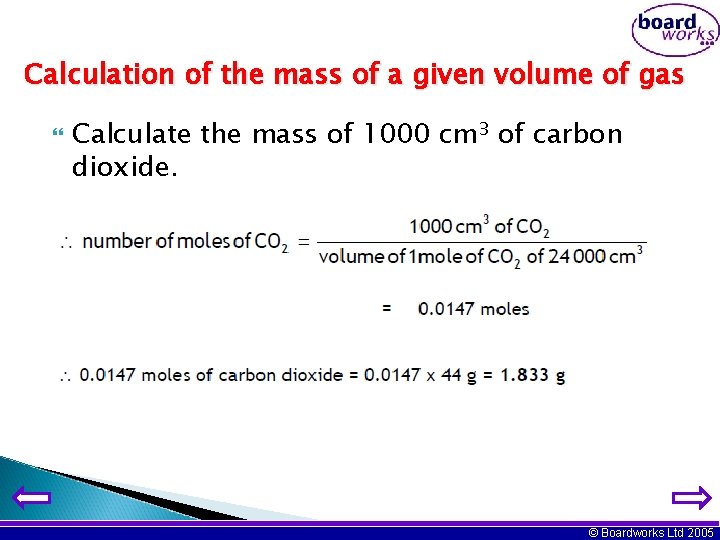
Calculation of the mass of a given volume of gas Calculate the mass of 1000 cm 3 of carbon dioxide. © Boardworks Ltd 2005

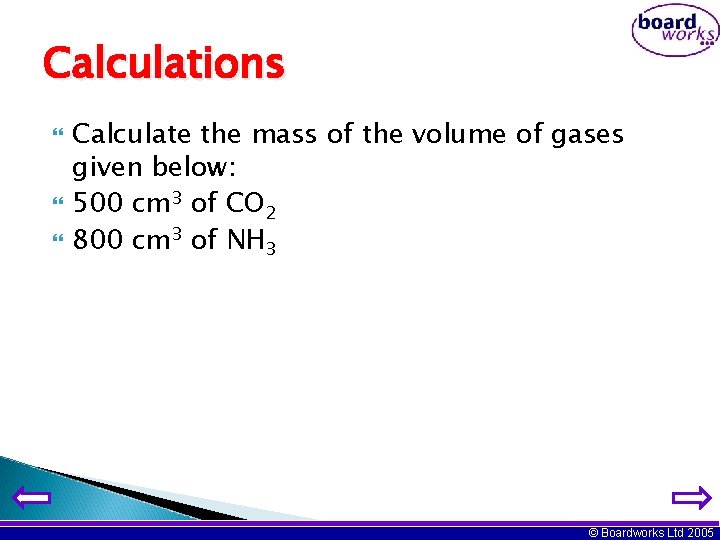
Calculations Calculate the mass of the volume of gases given below: 500 cm 3 of CO 2 800 cm 3 of NH 3 © Boardworks Ltd 2005

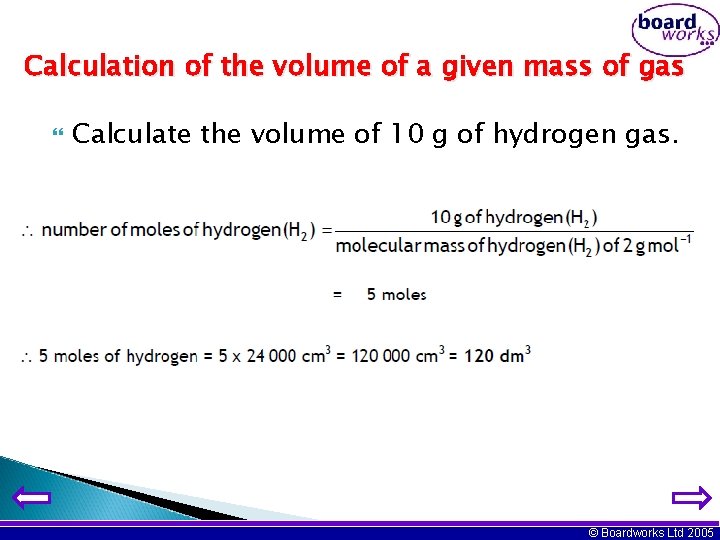
Calculation of the volume of a given mass of gas Calculate the volume of 10 g of hydrogen gas. © Boardworks Ltd 2005

Calculations Calculate the volume in cm 3 of the mass of gas given. 12 g of CO 2 25 g of NH 3 © Boardworks Ltd 2005

Calculations based on equations involving only gases © Boardworks Ltd 2005

Calculations based on equations involving only gases Assume that you have 10 cm 3 of the first named reactant and then calculate the volumes of all the gases involved in the equation. © Boardworks Ltd 2005

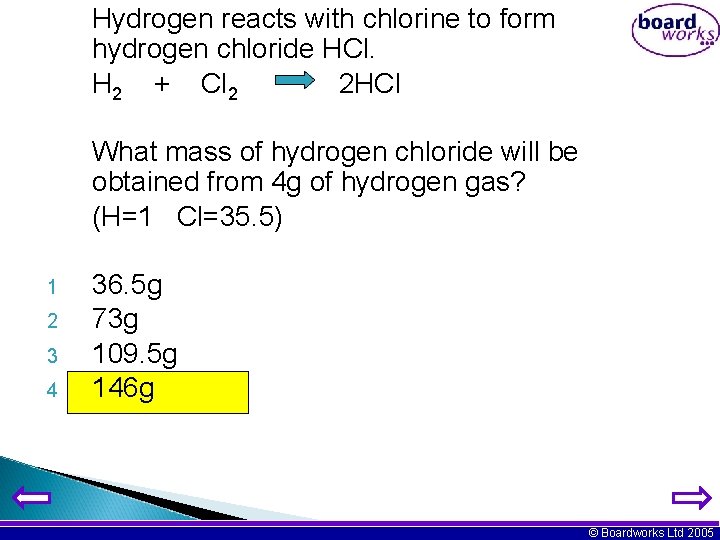
Hydrogen reacts with chlorine to form hydrogen chloride HCl. H 2 + Cl 2 2 HCl What mass of hydrogen chloride will be obtained from 4 g of hydrogen gas? (H=1 Cl=35. 5) 1 2 3 4 36. 5 g 73 g 109. 5 g 146 g © Boardworks Ltd 2005
 Amount of substance equations
Amount of substance equations Sequence series formula
Sequence series formula Example of specific topic
Example of specific topic Narrow
Narrow Formulae for moles
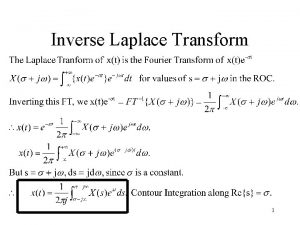
Formulae for moles Inverse laplace formulae
Inverse laplace formulae Transposing equations
Transposing equations Suvat formulas
Suvat formulas Dr frost equations
Dr frost equations Note c d e f g
Note c d e f g Dr frost quadratic graphs
Dr frost quadratic graphs Dr frost maths sequences
Dr frost maths sequences What is high art and low art
What is high art and low art Meaning simple interest
Meaning simple interest Suvat formulae
Suvat formulae Hc human capital
Hc human capital Topic 3 the mathematics of formulas and equations
Topic 3 the mathematics of formulas and equations Talking about amounts
Talking about amounts Ratio of amounts
Ratio of amounts Line/strip search pattern
Line/strip search pattern Problem 8-3 extending amounts across the work sheet
Problem 8-3 extending amounts across the work sheet How to determine if a single replacement reaction occurs
How to determine if a single replacement reaction occurs Calculating customs bond amounts
Calculating customs bond amounts Section 5 topic 3 solving quadratic equations by factoring
Section 5 topic 3 solving quadratic equations by factoring Topic 9 solving quadratic equations
Topic 9 solving quadratic equations Section 5 topic 3 solving quadratic equations by factoring
Section 5 topic 3 solving quadratic equations by factoring Rstuv
Rstuv Section 2 topic 1 linear equations in one variable part 1
Section 2 topic 1 linear equations in one variable part 1 Polar and rectangular forms of equations
Polar and rectangular forms of equations Translate word equations to chemical equations
Translate word equations to chemical equations Topic sentence
Topic sentence Topic 15 periods authors and genres
Topic 15 periods authors and genres Topic and closing sentences
Topic and closing sentences How to write a topic sentence
How to write a topic sentence