TOPIC Predicting amounts of reagents needed or amounts

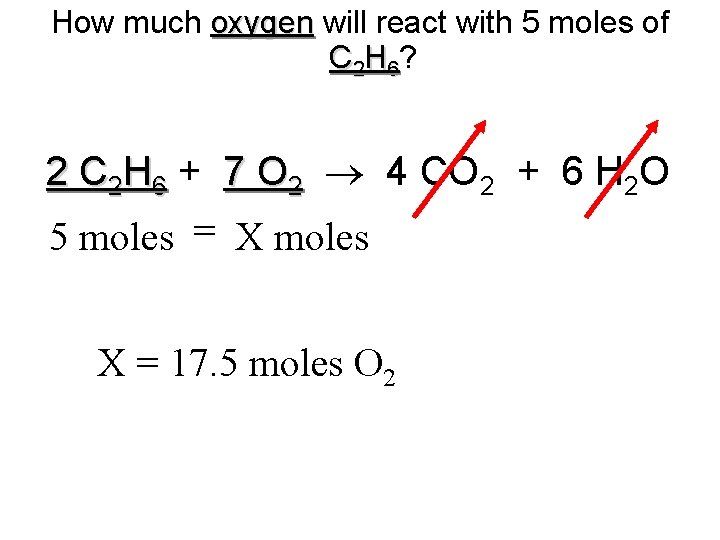
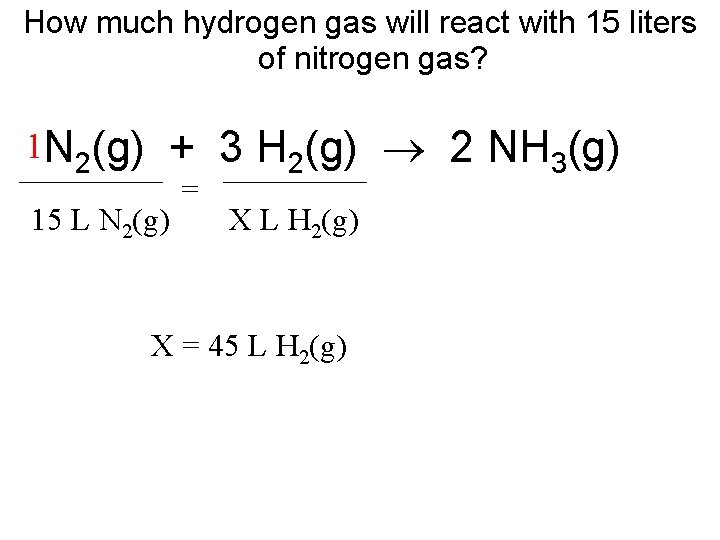
- Slides: 12

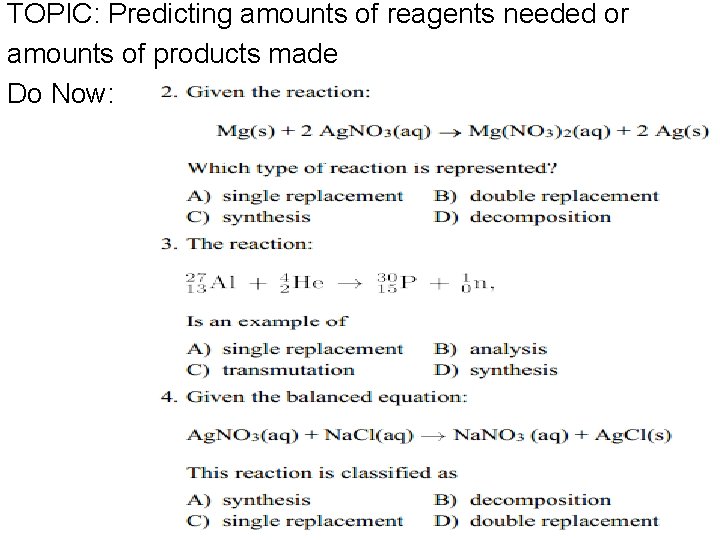
TOPIC: Predicting amounts of reagents needed or amounts of products made Do Now:

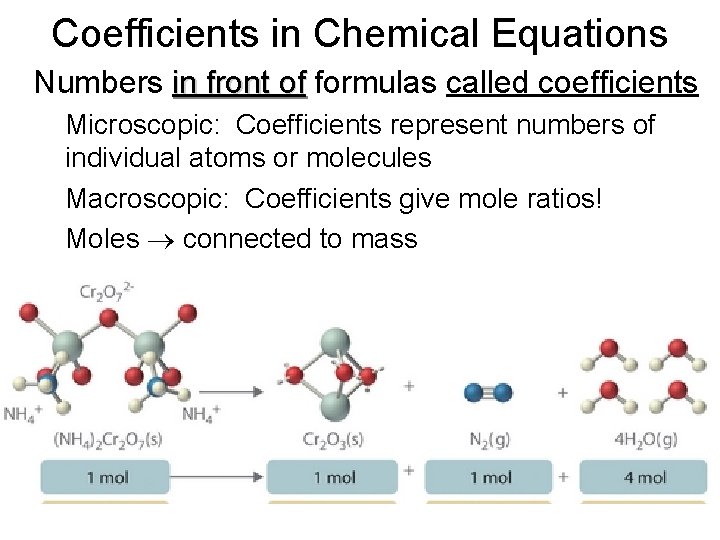
Coefficients in Chemical Equations • Numbers in front of formulas called coefficients – Microscopic: Coefficients represent numbers of individual atoms or molecules – Macroscopic: Coefficients give mole ratios! – Moles connected to mass

Coefficients in Balanced Equations MOLE-MOLE word problems: convert from moles of one substance to moles of another substance

2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O How much CO 2 will be produced if 4 moles of C 2 H 6 are consumed? 1) Start with balanced chemical equation 2) Problem will ask how many moles/liters given will yield certain amount something else 3) Use a proportion to compare old mole/liter amounts to the new mole/liter amounts

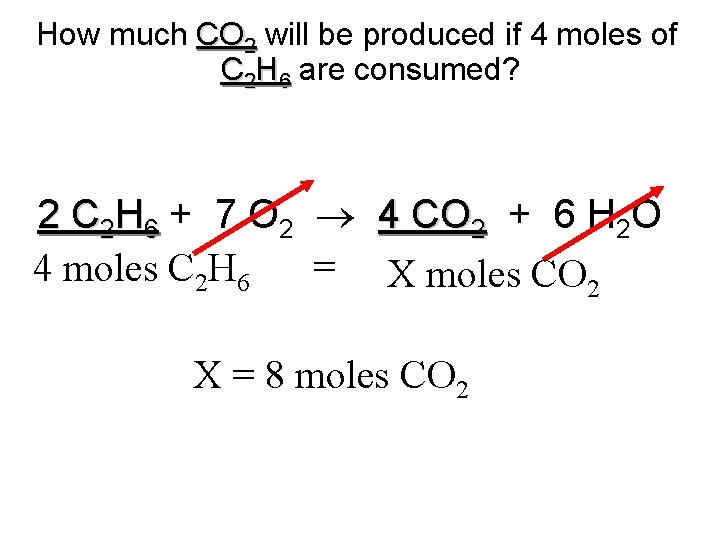
How much CO 2 will be produced if 4 moles of C 2 H 6 are consumed? 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O = X moles CO 2 4 moles C 2 H 6 X = 8 moles CO 2

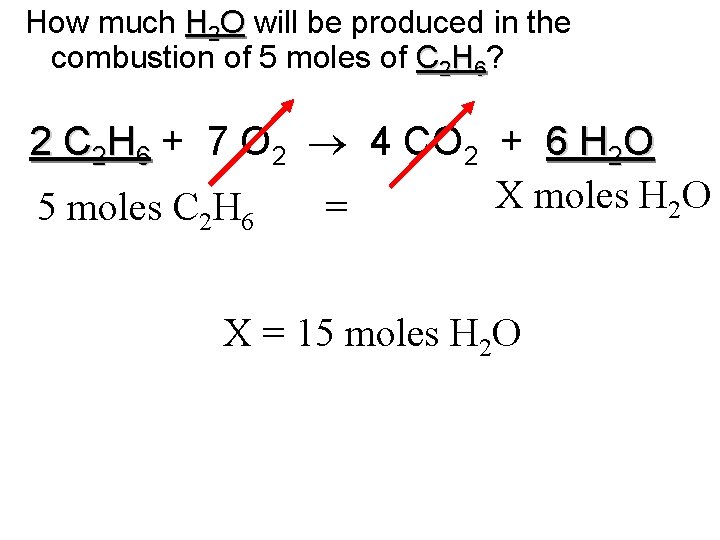
How much H 2 O will be produced in the combustion of 5 moles of C 2 H 6? 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O X moles H 2 O 5 moles C H = 2 6 X = 15 moles H 2 O
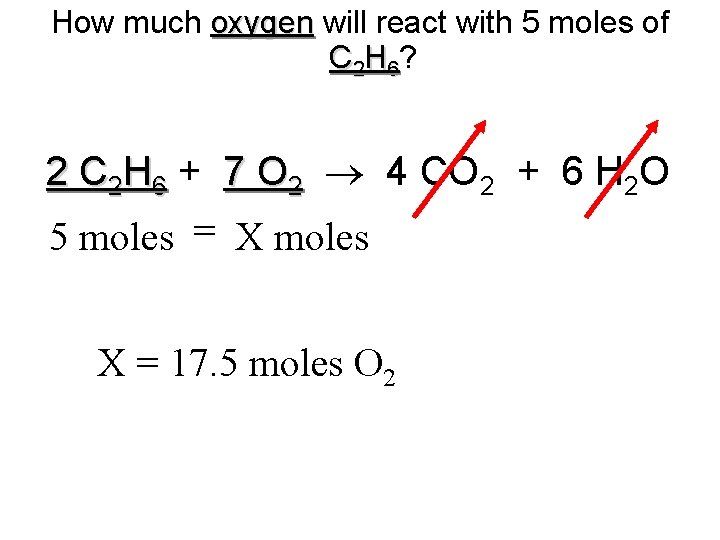
How much oxygen will react with 5 moles of C 2 H 6? 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O 5 moles = X moles X = 17. 5 moles O 2

Gas-Phase Equations • coefficients in equations represent ratio of volumes of gases involved in rxn • volume-volume word problems: rxns where ALL reactants & products are gases • volume unit (liter/milliliter) doesn’t matter as long as constant throughout
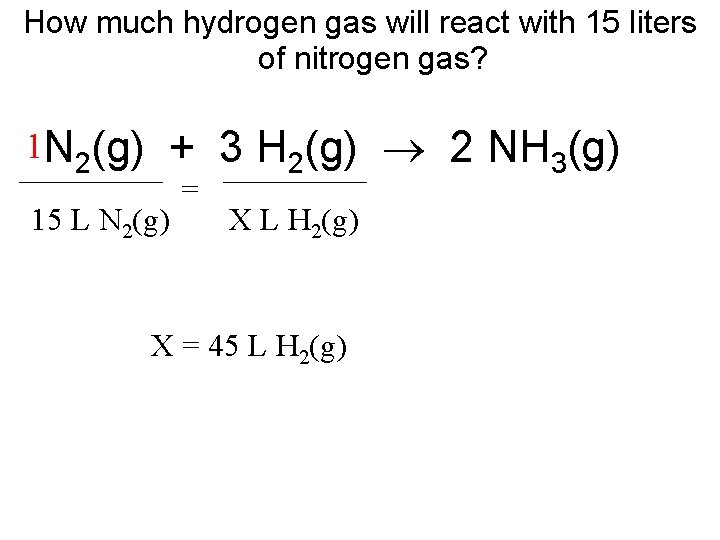
How much hydrogen gas will react with 15 liters of nitrogen gas? + 3 H (g) 2 NH (g) 2 3 _________ 1 N 2(g) 15 L N 2(g) = X L H 2(g) X = 45 L H 2(g)

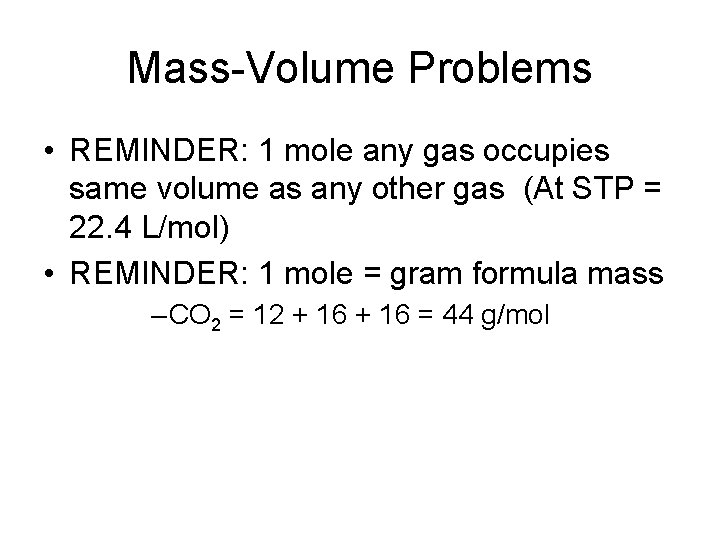
Mass-Volume Problems • REMINDER: 1 mole any gas occupies same volume as any other gas (At STP = 22. 4 L/mol) • REMINDER: 1 mole = gram formula mass – CO 2 = 12 + 16 = 44 g/mol

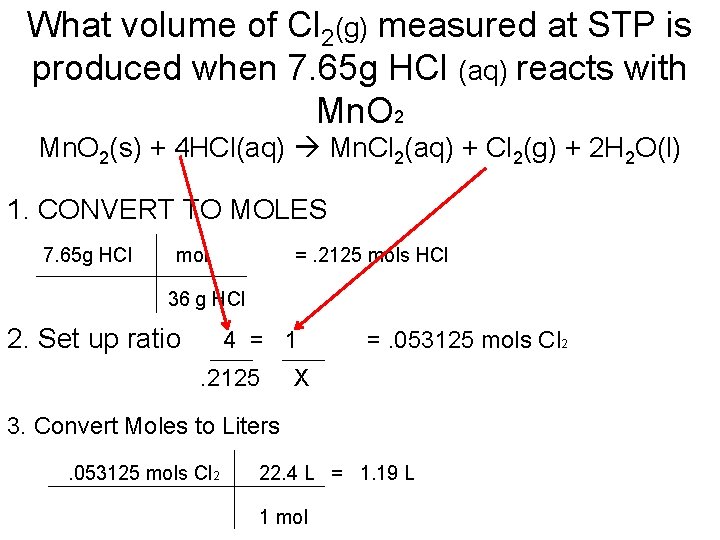
What volume of Cl 2(g) measured at STP is produced when 7. 65 g HCl (aq) reacts with Mn. O 2(s) + 4 HCl(aq) Mn. Cl 2(aq) + Cl 2(g) + 2 H 2 O(l) 1. CONVERT TO MOLES 7. 65 g HCl mol =. 2125 mols HCl 36 g HCl 2. Set up ratio 4 = 1. 2125 =. 053125 mols Cl 2 X 3. Convert Moles to Liters. 053125 mols Cl 2 22. 4 L = 1. 19 L 1 mol

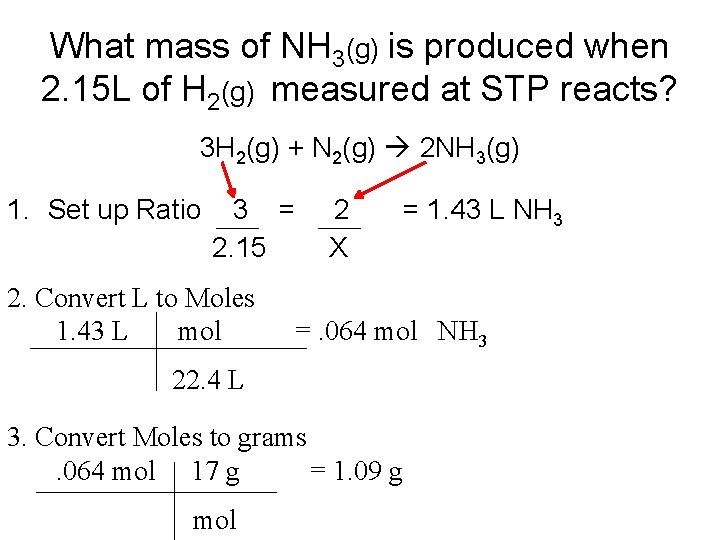
What mass of NH 3(g) is produced when 2. 15 L of H 2(g) measured at STP reacts? 3 H 2(g) + N 2(g) 2 NH 3(g) 1. Set up Ratio 3 = 2. 15 2. Convert L to Moles 1. 43 L mol 2 X =. 064 mol NH 3 22. 4 L 3. Convert Moles to grams. 064 mol 17 g = 1. 09 g mol = 1. 43 L NH 3