Stoichiometry Predicting amounts of reagents needed or amounts







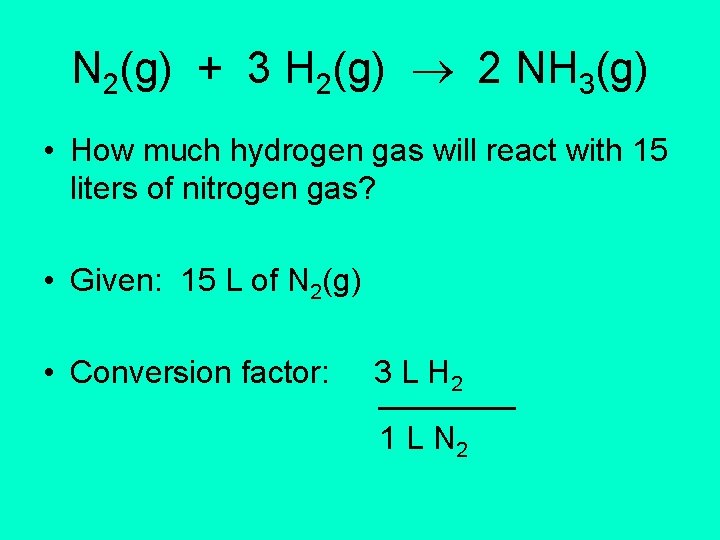








- Slides: 30

Stoichiometry Predicting amounts of reagents needed or amounts of products made

Stoichiometry • Composition stoichiometry – Mass relationships of elements in compounds • Reaction stoichiometry – Mass relationships between participants in a chemical reaction • Must begin all stoichiometry problems with a BALANCED chemical equation.

Conservation of Charge • Balanced chemical equation must be balanced for both mass and charge. • Total charge on reactant side must equal total charge on product side. • So far, most of the equations you have seen are neutral on each side, but this is not always the case.

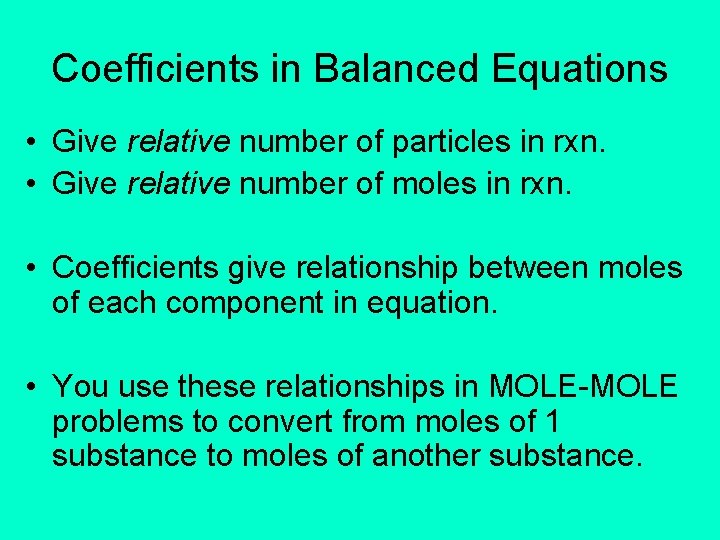
Coefficients in Balanced Equations • Give relative number of particles in rxn. • Give relative number of moles in rxn. • Coefficients give relationship between moles of each component in equation. • You use these relationships in MOLE-MOLE problems to convert from moles of 1 substance to moles of another substance.

Stoichiometry Problems • Come in several flavors. • Start with the simplest: mole-mole • Given: amount of one substance in moles • Unknown: amount of some other substance in moles

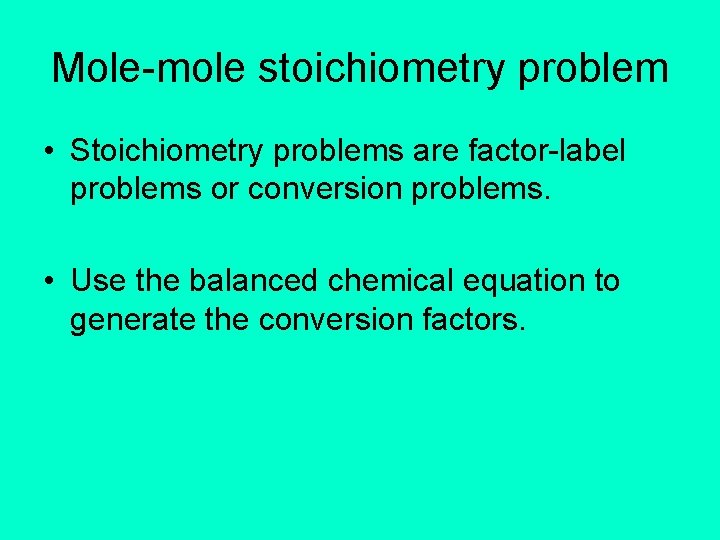
Mole-mole stoichiometry problem • Stoichiometry problems are factor-label problems or conversion problems. • Use the balanced chemical equation to generate the conversion factors.

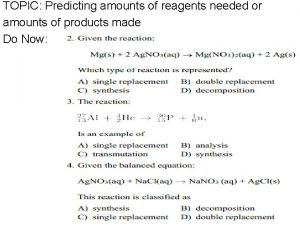
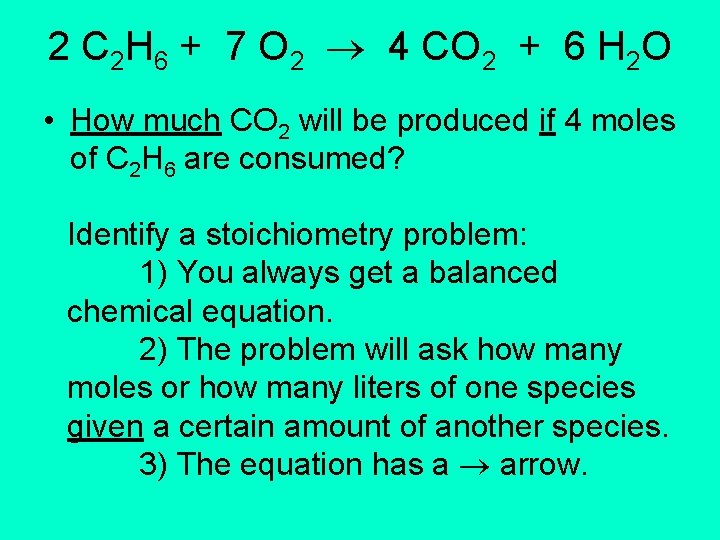
2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O • How much CO 2 will be produced if 4 moles of C 2 H 6 are consumed? Identify a stoichiometry problem: 1) You always get a balanced chemical equation. 2) The problem will ask how many moles or how many liters of one species given a certain amount of another species. 3) The equation has a arrow.

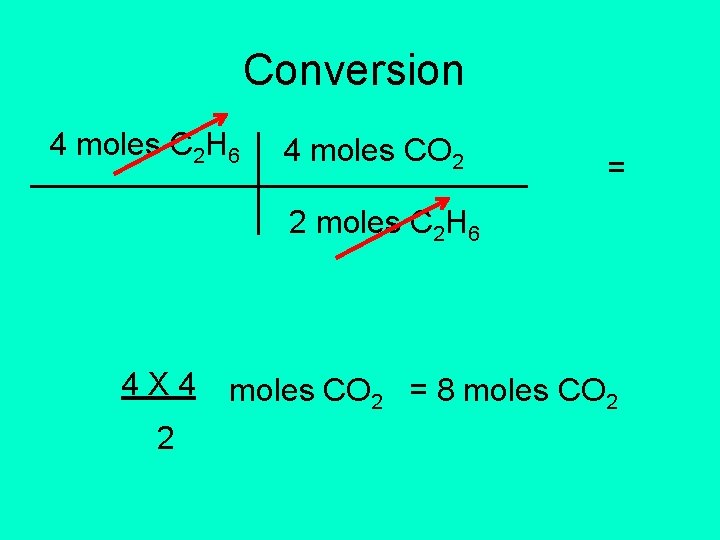
2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O • How much CO 2 will be produced if 4 moles of C 2 H 6 are consumed? • Identify given: 4 moles of C 2 H 6. • Identify conversion factor from equation: 4 moles CO 2 2 moles C 2 H 6

Conversion 4 moles C 2 H 6 4 moles CO 2 = 2 moles C 2 H 6 4 X 4 2 moles CO 2 = 8 moles CO 2

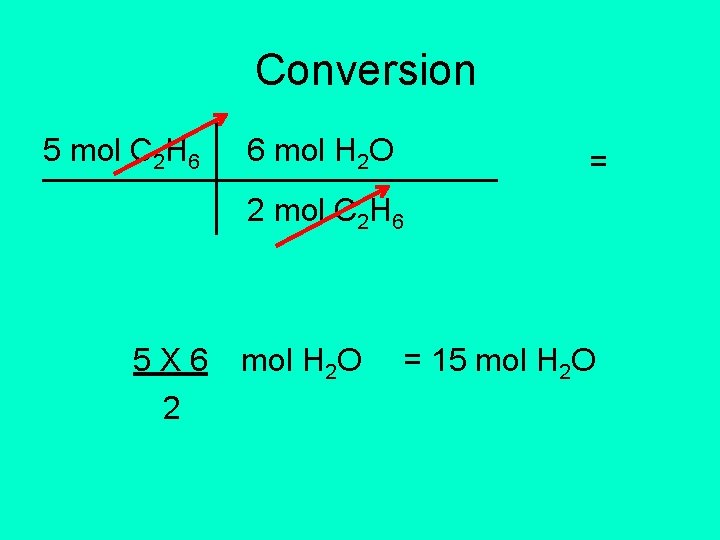
2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O • How much H 2 O will be produced in the combustion of 5 moles of C 2 H 6? • Given: 5 mol C 2 H 6 • Conversion factor: 6 mol H 2 O 2 mol C 2 H 6

Conversion 5 mol C 2 H 6 6 mol H 2 O = 2 mol C 2 H 6 5 X 6 2 mol H 2 O = 15 mol H 2 O

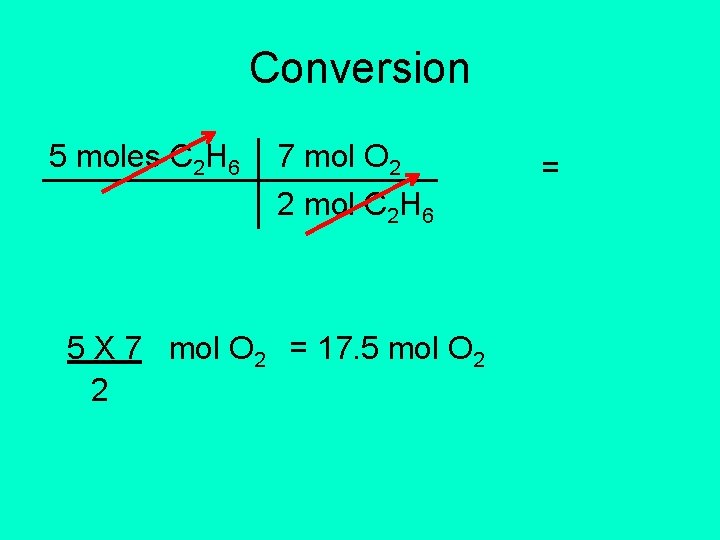
2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O • How much oxygen will react with 5 moles of C 2 H 6? • Given: 5 moles C 2 H 6 • Conversion factor: 7 mol O 2 2 mol C 2 H 6

Conversion 5 moles C 2 H 6 7 mol O 2 2 mol C 2 H 6 5 X 7 mol O 2 = 17. 5 mol O 2 2 =

Other flavors of problems • Other types of stoichiometry problems include: • Volume-Volume (for gas phase only) • Mole-mass or mass-mole • Mass-Mass

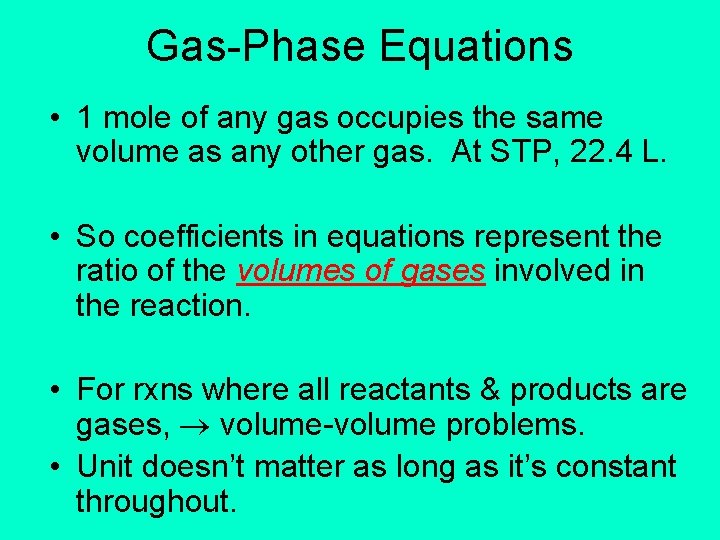
Gas-Phase Equations • 1 mole of any gas occupies the same volume as any other gas. At STP, 22. 4 L. • So coefficients in equations represent the ratio of the volumes of gases involved in the reaction. • For rxns where all reactants & products are gases, volume-volume problems. • Unit doesn’t matter as long as it’s constant throughout.
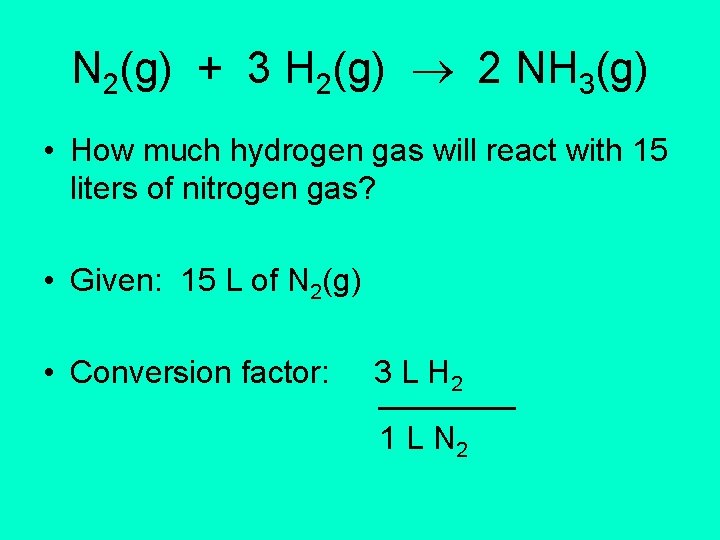
N 2(g) + 3 H 2(g) 2 NH 3(g) • How much hydrogen gas will react with 15 liters of nitrogen gas? • Given: 15 L of N 2(g) • Conversion factor: 3 L H 2 1 L N 2

Conversion 15 L N 2(g) 3 L H 2 = 1 L N 2 15 X 3 L H 2 1 = 45 L H 2

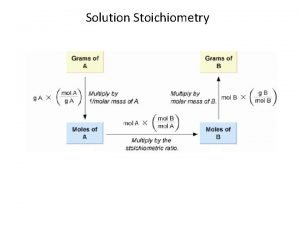
Other types of stoich problems • General strategy: 1. Convert given into moles 2. Perform stoichiometric calculation using mole ratios from balanced equation 3. Convert to desired unit

A word about …

Reactions in aqueous solution • Many reactions, esp. many double replacement reactions, occur in water. • What happens when substances dissolve in water? • Depends on if they are ionic or covalent.

Dissolving • Covalent substance – sugar or C 6 H 12 O 6 • C 6 H 12 O 6(s) C 6 H 12 O 6(aq) • The sugar molecules are spread out among the water molecules.

Dissolving • Ionic substance – table salt or Na. Cl • Na. Cl(s) Na+(aq) + Cl-(aq) • The ions are spread out among the water molecules.

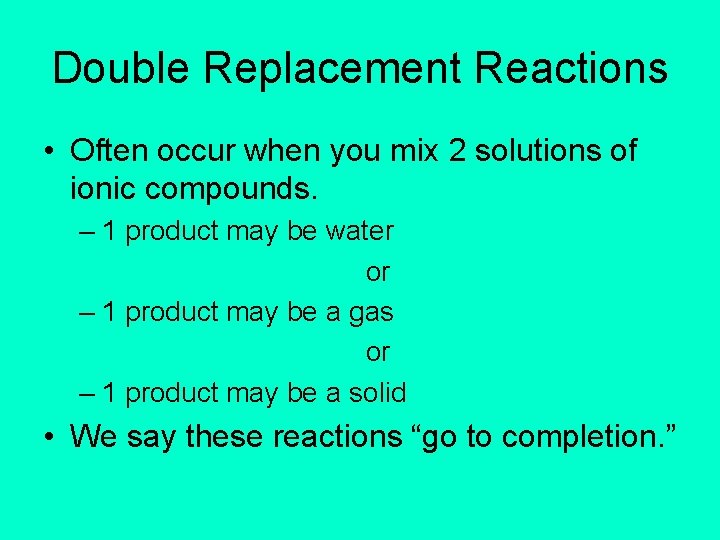
Double Replacement Reactions • Often occur when you mix 2 solutions of ionic compounds. – 1 product may be water or – 1 product may be a gas or – 1 product may be a solid • We say these reactions “go to completion. ”

Reactions producing Solids • • Precipitation: the opposite of dissolving! What do you see in the following clips: S 1043. mov S 1045. mov S 1046. mov S 1050. mov S 1057. mov S 1058. mov and S 1060. mov

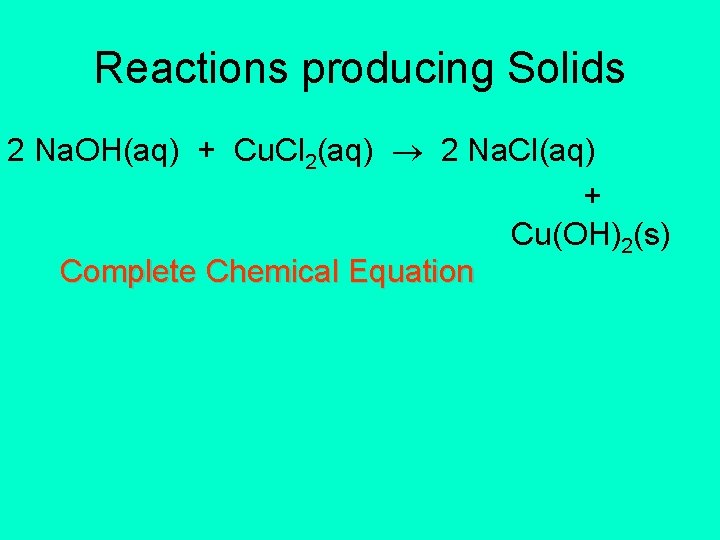
Reactions producing Solids 2 Na. OH(aq) + Cu. Cl 2(aq) 2 Na. Cl(aq) + Cu(OH)2(s) Complete Chemical Equation

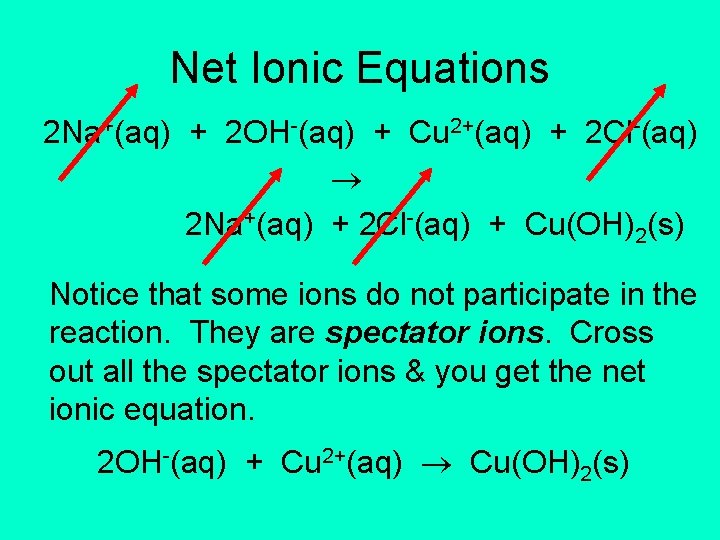
Complete Ionic Equations 2 Na+(aq) + 2 OH-(aq) + Cu 2+(aq) + 2 Cl-(aq) 2 Na+(aq) + 2 Cl-(aq) + Cu(OH)2(s) Substances that are ions in solution are written as ions in solution.

Net Ionic Equations 2 Na+(aq) + 2 OH-(aq) + Cu 2+(aq) + 2 Cl-(aq) 2 Na+(aq) + 2 Cl-(aq) + Cu(OH)2(s) Notice that some ions do not participate in the reaction. They are spectator ions. Cross out all the spectator ions & you get the net ionic equation. 2 OH-(aq) + Cu 2+(aq) Cu(OH)2(s)

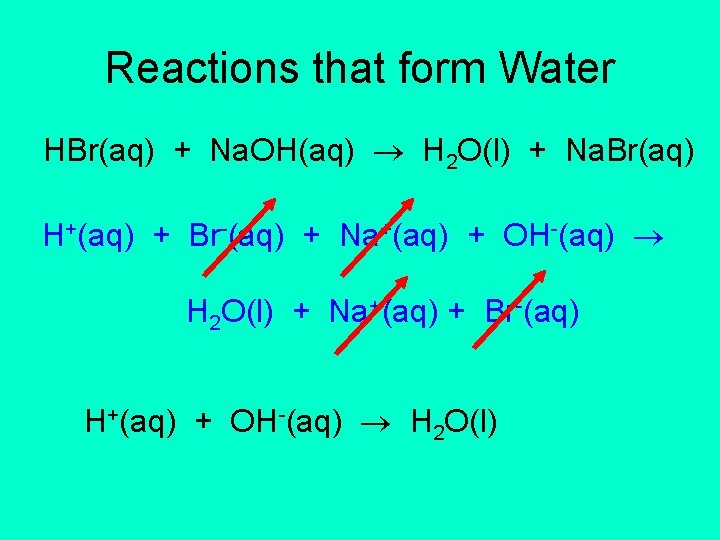
Reactions that form Water HBr(aq) + Na. OH(aq) H 2 O(l) + Na. Br(aq) H+(aq) + Br-(aq) + Na+(aq) + OH-(aq) H 2 O(l) + Na+(aq) + Br-(aq) H+(aq) + OH-(aq) H 2 O(l)

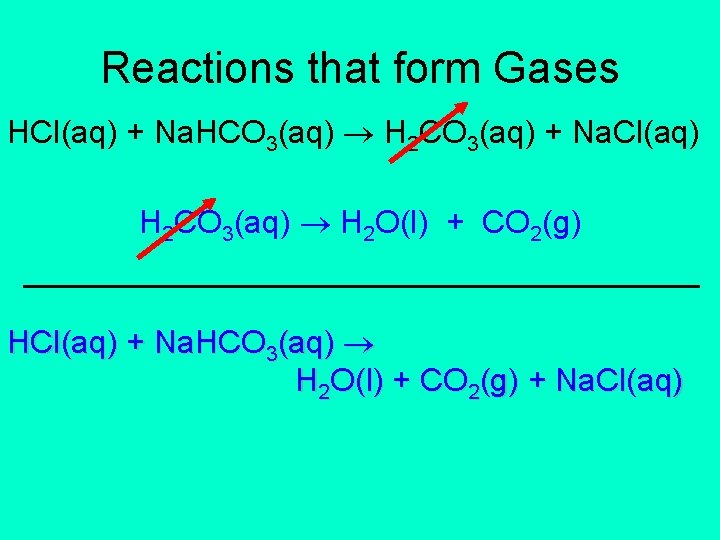
Reactions that form Gases HCl(aq) + Na. HCO 3(aq) H 2 CO 3(aq) + Na. Cl(aq) H 2 CO 3(aq) H 2 O(l) + CO 2(g) ___________________ HCl(aq) + Na. HCO 3(aq) H 2 O(l) + CO 2(g) + Na. Cl(aq)

Reactions that form Gases HCl(aq) + Na. HCO 3(aq) H 2 O(l) + CO 2(g) + Na. Cl(aq) H+(aq) + Cl-(aq) + Na+(aq) + HCO 3 -(aq) H 2 O(l) + CO 2(g) + Na+(aq) + Cl-(aq) H+(aq) + HCO 3 -(aq) H 2 O(l) + CO 2(g)
 How to determine if a single replacement reaction occurs
How to determine if a single replacement reaction occurs What are the drawbacks when using benzidine reagent?
What are the drawbacks when using benzidine reagent? Oxidation of alcohol to aldehyde
Oxidation of alcohol to aldehyde How to calculate limiting reactant
How to calculate limiting reactant Alkaloidal reagents denature proteins
Alkaloidal reagents denature proteins Urease test bacteria
Urease test bacteria Double stuffed oreo lab answers
Double stuffed oreo lab answers Hydrogen halide example
Hydrogen halide example Five elements of quality control in blood banking
Five elements of quality control in blood banking Kinetics flotation reagents
Kinetics flotation reagents Hypochrmic
Hypochrmic High-quality reagents
High-quality reagents Synthesis reaction predicting products
Synthesis reaction predicting products Communicating in science process skills
Communicating in science process skills Predicting spontaneity
Predicting spontaneity Braden scale for predicting pressure sore risk
Braden scale for predicting pressure sore risk Predicting molecular polarity
Predicting molecular polarity Predicting content in listening
Predicting content in listening How to calculate bond angle
How to calculate bond angle Combination reaction example
Combination reaction example Predicting fraud
Predicting fraud Explain what is meant by selective use of the ipde process
Explain what is meant by selective use of the ipde process Science process skills predicting
Science process skills predicting Ten ways to untwist your thinking
Ten ways to untwist your thinking Apa itu previewing and predicting
Apa itu previewing and predicting Predicting and naming ionic compounds
Predicting and naming ionic compounds Predicting products of chemical reactions
Predicting products of chemical reactions The evolution of crm is reporting analyzing and predicting
The evolution of crm is reporting analyzing and predicting Predicting single replacement reactions
Predicting single replacement reactions Braden scale for predicting pressure sore risk
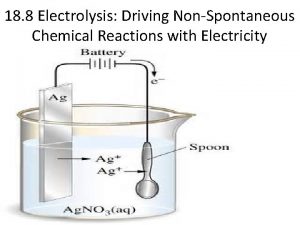
Braden scale for predicting pressure sore risk Predicting products of electrolysis
Predicting products of electrolysis