CHAPTER 3 CHEMICAL FORMULAE EQUATIONS Chemical Formulae 4








- Slides: 23

CHAPTER 3: CHEMICAL FORMULAE & EQUATIONS Chemical Formulae 4 S 1 Tuesday, 1/3/11 10. 45 – 11. 55 am

Instead of writing long description of chemicals or substances, chemists write their chemical formulae. For example, hydrogen gas is written

So, what is a chemical formula? ? ?

Chemical formula = a representation of a chemical substance using letters for atoms & subscript numbers to show the numbers of each type of atoms that are present in the substance

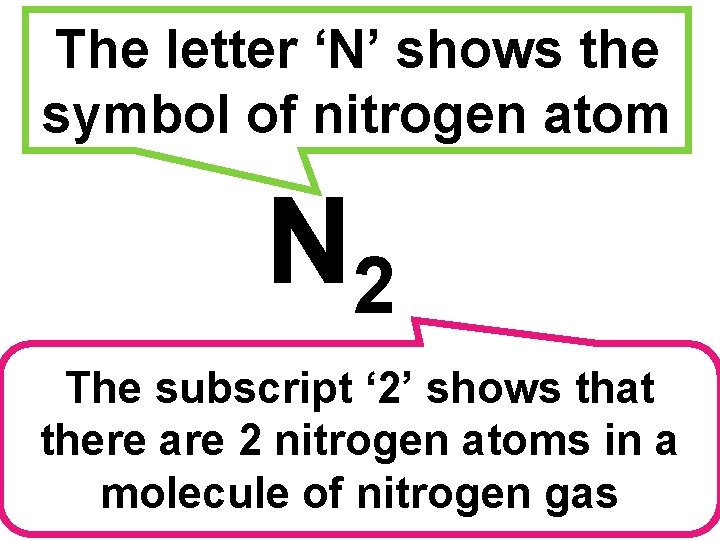
The letter ‘N’ shows the symbol of nitrogen atom N 2 The subscript ‘ 2’ shows that there are 2 nitrogen atoms in a molecule of nitrogen gas

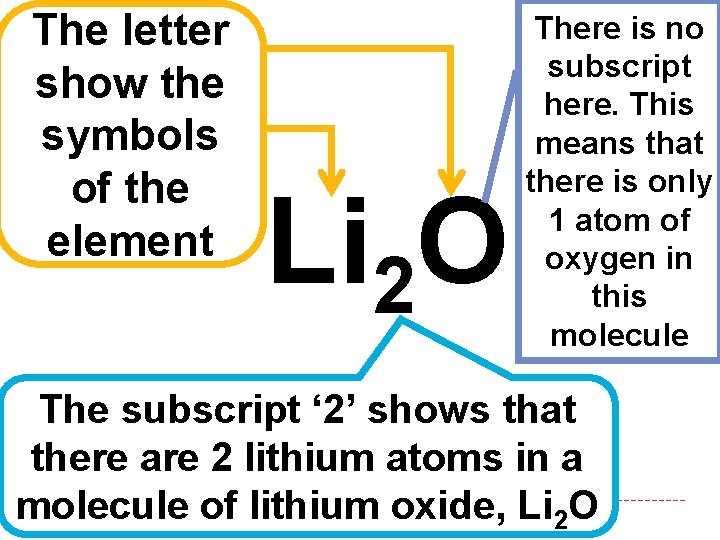
The letter show the symbols of the element Li 2 O There is no subscript here. This means that there is only 1 atom of oxygen in this molecule The subscript ‘ 2’ shows that there are 2 lithium atoms in a molecule of lithium oxide, Li 2 O

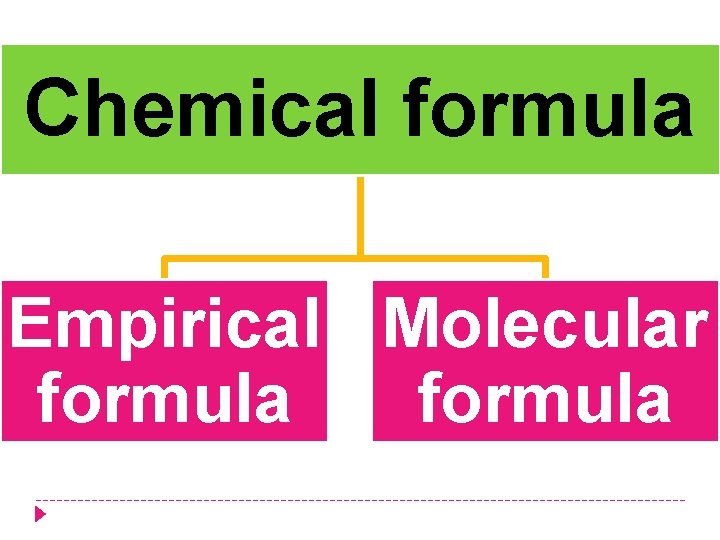
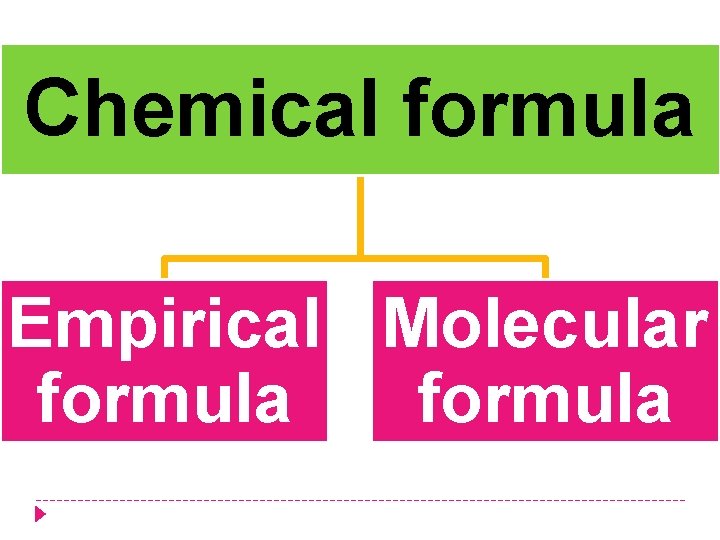
Chemical formula Empirical Molecular formula

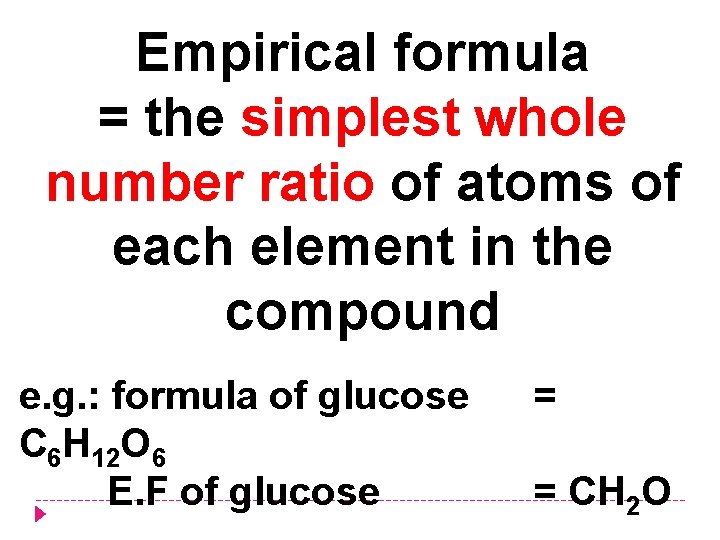
Empirical formula = the simplest whole number ratio of atoms of each element in the compound e. g. : formula of glucose C 6 H 12 O 6 E. F of glucose = = CH 2 O

Determining empirical formula of a compound

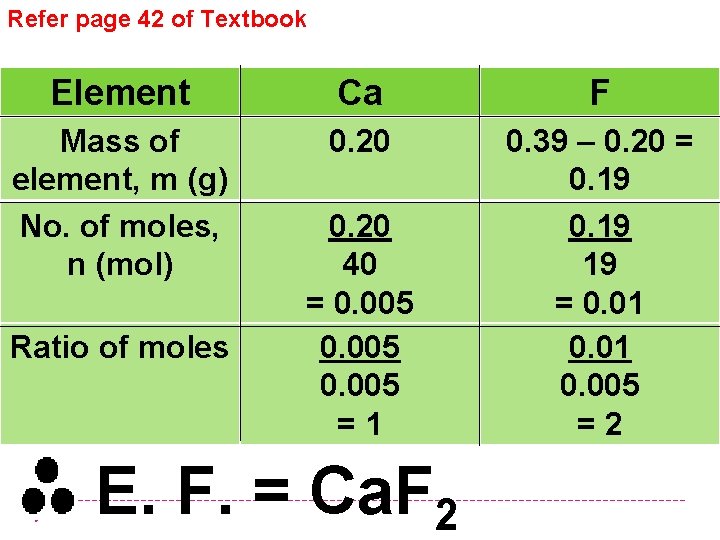
Refer page 42 of Textbook Element Ca F Mass of element, m (g) No. of moles, n (mol) 0. 20 0. 39 – 0. 20 = 0. 19 19 = 0. 01 0. 005 =2 Ratio of moles 0. 20 40 = 0. 005 =1 E. F. = Ca. F 2

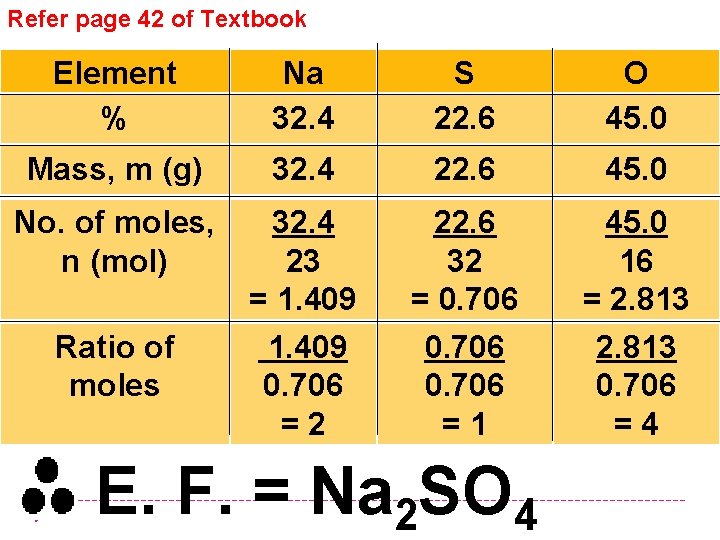
Refer page 42 of Textbook Element % Na 32. 4 S 22. 6 O 45. 0 Mass, m (g) 32. 4 22. 6 45. 0 No. of moles, n (mol) 32. 4 23 = 1. 409 0. 706 =2 22. 6 32 = 0. 706 =1 45. 0 16 = 2. 813 0. 706 =4 Ratio of moles E. F. = Na 2 SO 4

Try Q 3 of Work This Out 3. 7 Calculation page 42

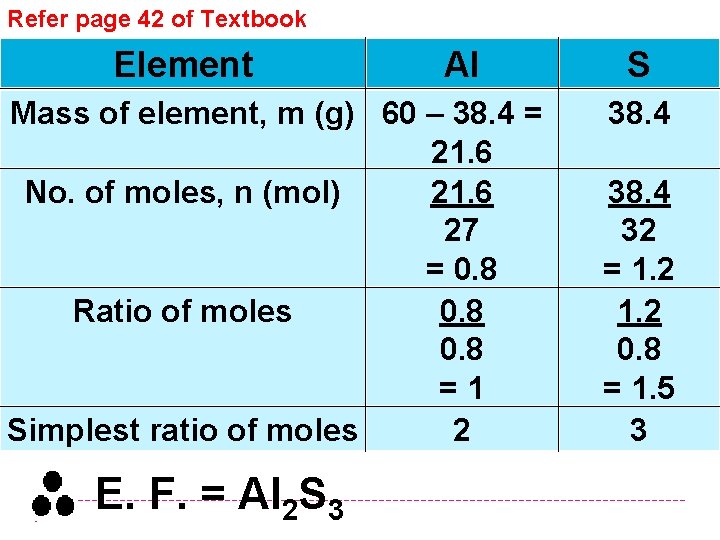
Refer page 42 of Textbook Element Al Mass of element, m (g) 60 – 38. 4 = 21. 6 No. of moles, n (mol) 21. 6 27 = 0. 8 Ratio of moles 0. 8 =1 Simplest ratio of moles 2 E. F. = Al 2 S 3 S 38. 4 32 = 1. 2 0. 8 = 1. 5 3

Chemical formula Empirical Molecular formula

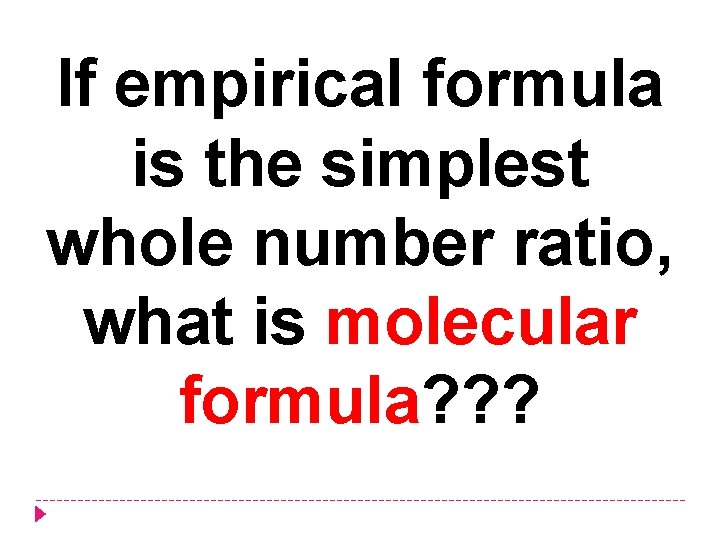
If empirical formula is the simplest whole number ratio, what is molecular formula? ? ?

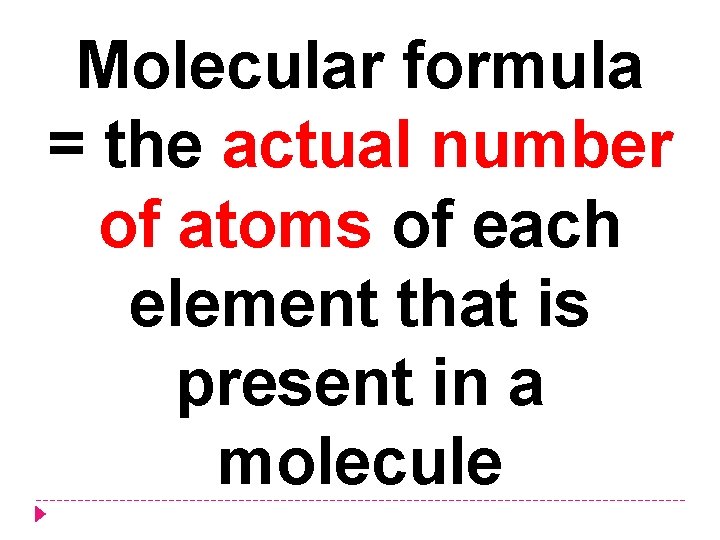
Molecular formula = the actual number of atoms of each element that is present in a molecule

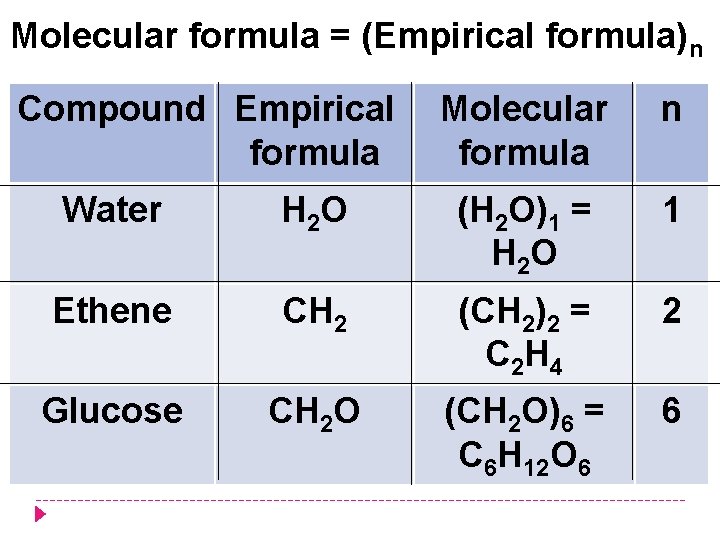
Molecular formula = (Empirical formula)n Compound Empirical formula Molecular formula n Water H 2 O (H 2 O)1 = H 2 O 1 Ethene CH 2 (CH 2)2 = C 2 H 4 2 Glucose CH 2 O (CH 2 O)6 = C 6 H 12 O 6 6

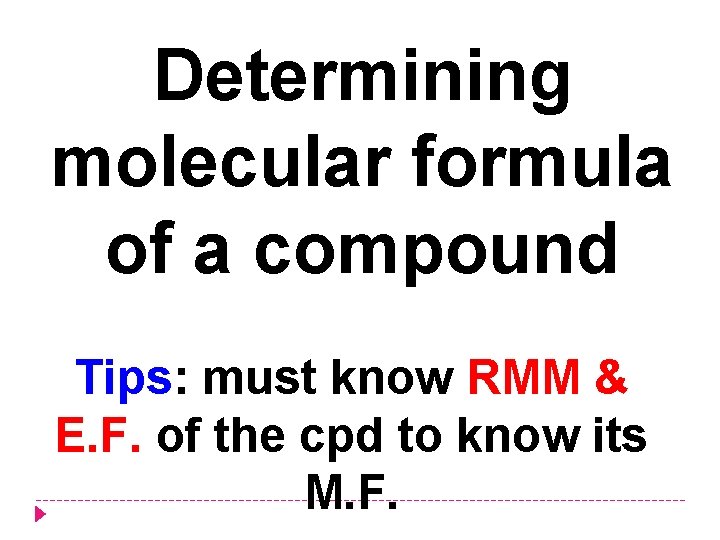
Determining molecular formula of a compound Tips: must know RMM & E. F. of the cpd to know its M. F.

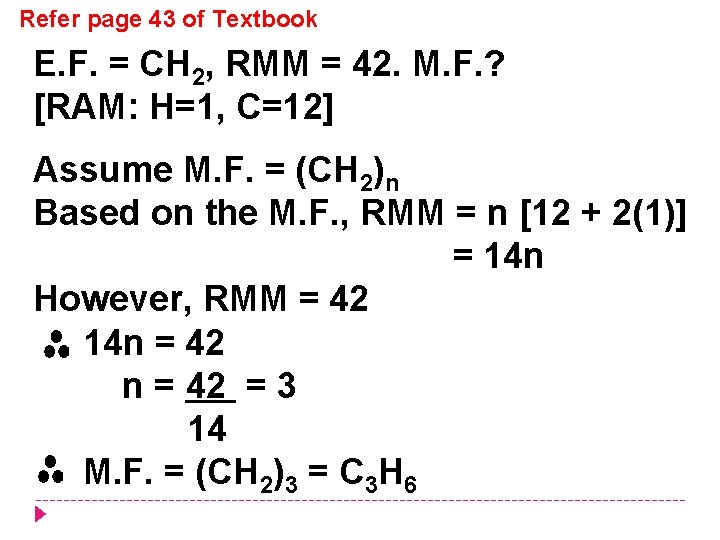
Refer page 43 of Textbook E. F. = CH 2, RMM = 42. M. F. ? [RAM: H=1, C=12] Assume M. F. = (CH 2)n Based on the M. F. , RMM = n [12 + 2(1)] = 14 n However, RMM = 42 14 n = 42 = 3 14 M. F. = (CH 2)3 = C 3 H 6

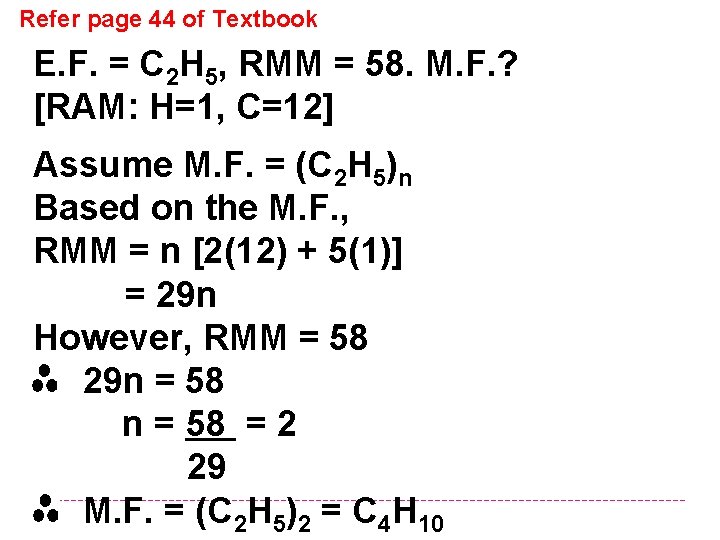
Refer page 44 of Textbook E. F. = C 2 H 5, RMM = 58. M. F. ? [RAM: H=1, C=12] Assume M. F. = (C 2 H 5)n Based on the M. F. , RMM = n [2(12) + 5(1)] = 29 n However, RMM = 58 29 n = 58 = 2 29 M. F. = (C 2 H 5)2 = C 4 H 10

Try Q 2 of Work This Out 3. 8 Calculation page 44

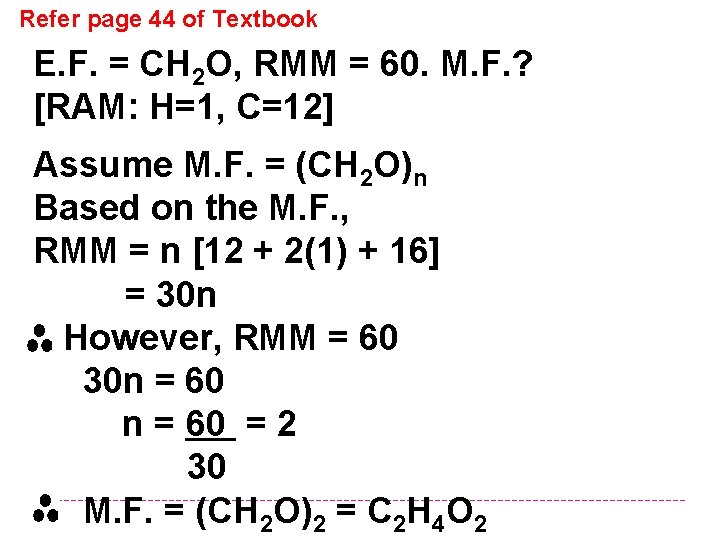
Refer page 44 of Textbook E. F. = CH 2 O, RMM = 60. M. F. ? [RAM: H=1, C=12] Assume M. F. = (CH 2 O)n Based on the M. F. , RMM = n [12 + 2(1) + 16] = 30 n However, RMM = 60 30 n = 60 = 2 30 M. F. = (CH 2 O)2 = C 2 H 4 O 2

REMINDER!!! Memorise the ionic formulae of cations and anions for the next lesson