CHAPTER 3 CHEMICAL FORMULAE EQUATIONS Relative Atomic Mass


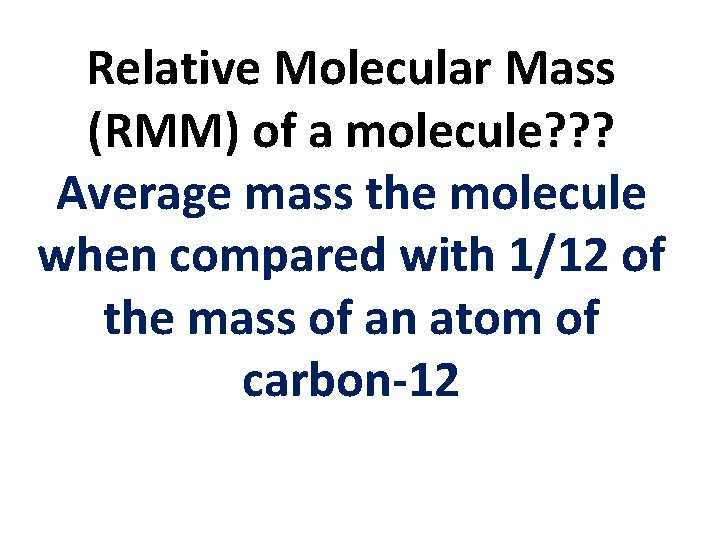





- Slides: 17

CHAPTER 3: CHEMICAL FORMULAE & EQUATIONS Relative Atomic Mass & Relative Molecular Mass 4 S 1 Monday, 7/2/2011 10. 45 – 11. 55 am

Electrochemistry Periodic Table of Elements Salts Acids & Bases Oxidation & Reduction Thermochemistry Rate of Reaction CHEMICAL FORMULAE AND

A. Relative Atomic Mass & Relative Molecular Mass B. The Mole and the Number of Particles C. The Mole and the Mass of Substances D. The Mole and the Volume of Gas E. Chemical Formulae F. Chemical Equations

What is the mass of an atom? Since an atom is very small, there is no machine in the world that can weigh it. Therefore, to determine the mass of an atom, scientists compare the mass of one atom of the element to the mass of one atom of carbon-12

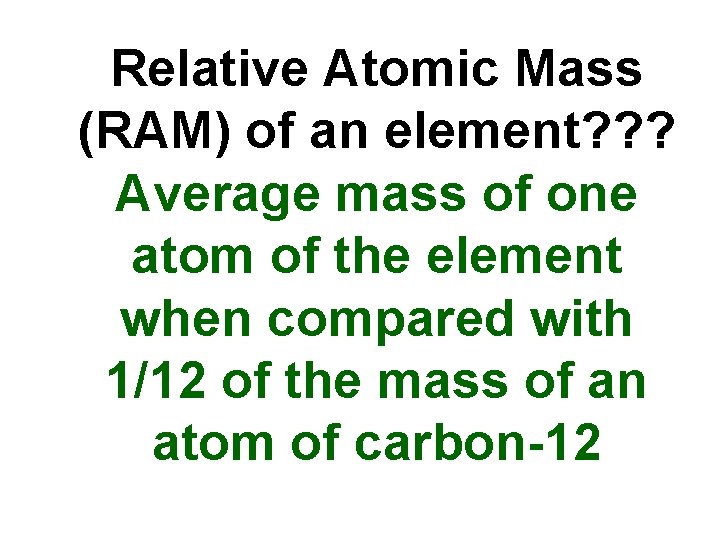
Relative Atomic Mass (RAM) of an element? ? ? Average mass of one atom of the element when compared with 1/12 of the mass of an atom of carbon-12

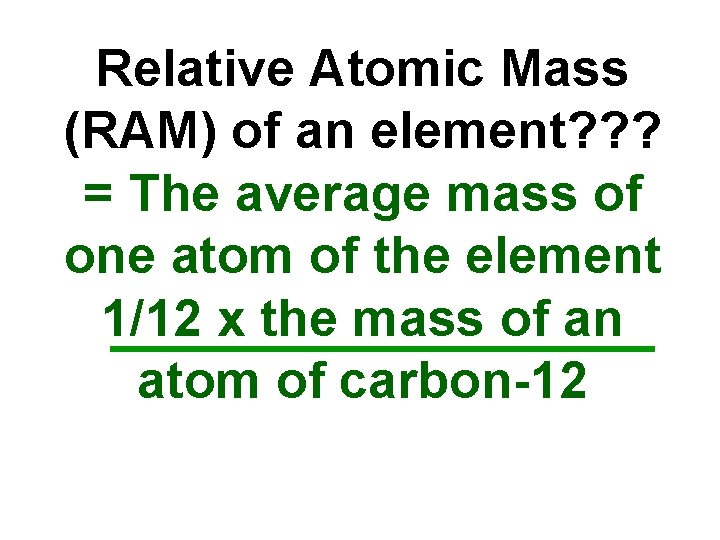
Relative Atomic Mass (RAM) of an element? ? ? = The average mass of one atom of the element 1/12 x the mass of an atom of carbon-12
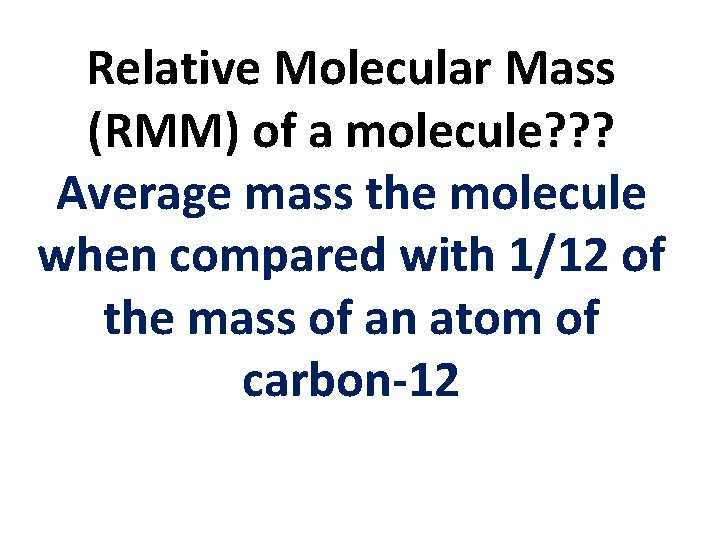
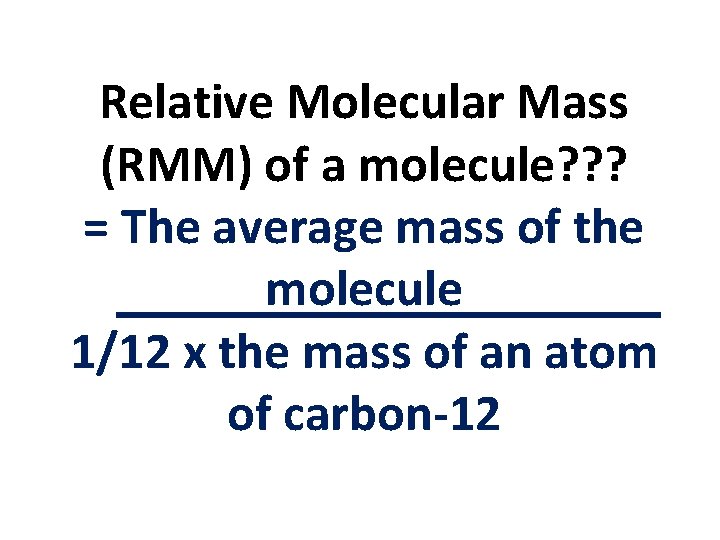
Relative Molecular Mass (RMM) of a molecule? ? ? Average mass the molecule when compared with 1/12 of the mass of an atom of carbon-12

Relative Molecular Mass (RMM) of a molecule? ? ? = The average mass of the molecule 1/12 x the mass of an atom of carbon-12

Why do we compare with carbon-12? (why is carbon-12 used as standard? ) • It is solid • It is easily handled • It is used as a reference standard in the mass spectrometer

Refer page 28 of your Textbook

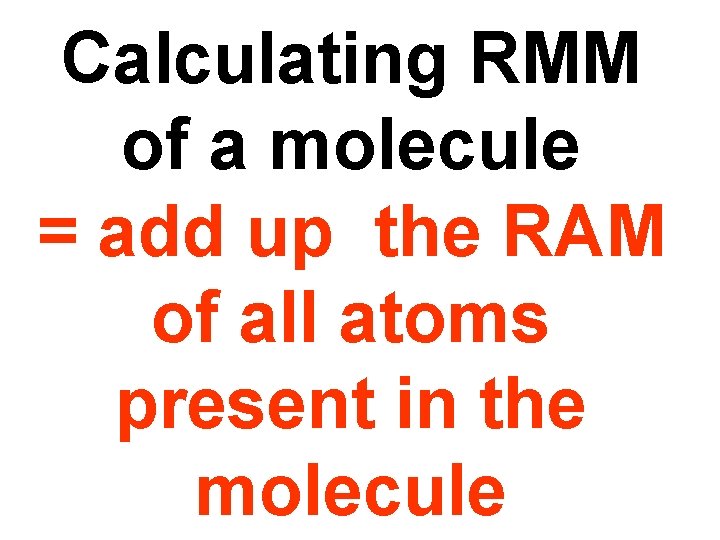
Calculating RMM of a molecule = add up the RAM of all atoms present in the molecule

Refer page 176 of your Textbook

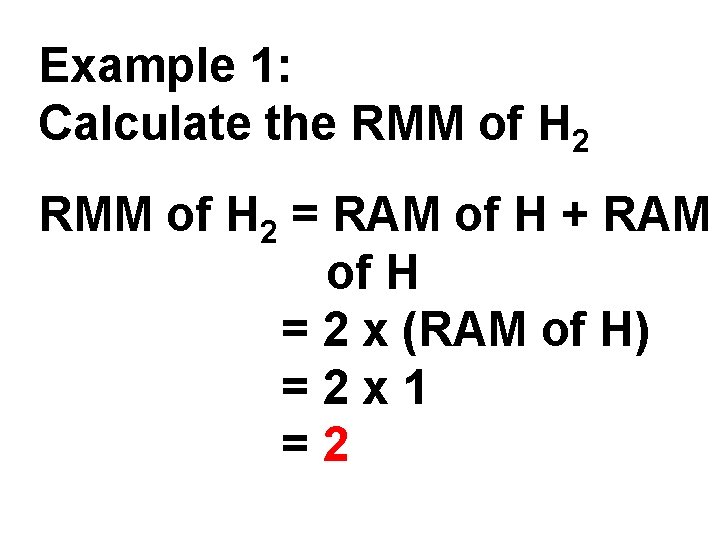
Example 1: Calculate the RMM of H 2 = RAM of H + RAM of H = 2 x (RAM of H) =2 x 1 =2

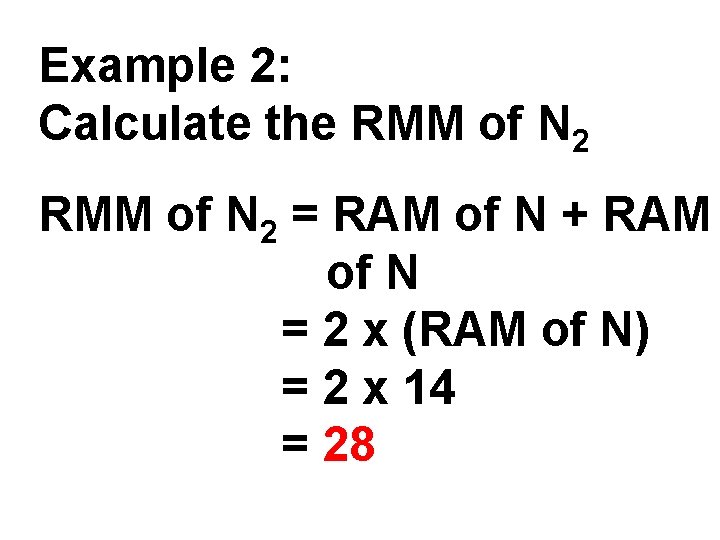
Example 2: Calculate the RMM of N 2 = RAM of N + RAM of N = 2 x (RAM of N) = 2 x 14 = 28

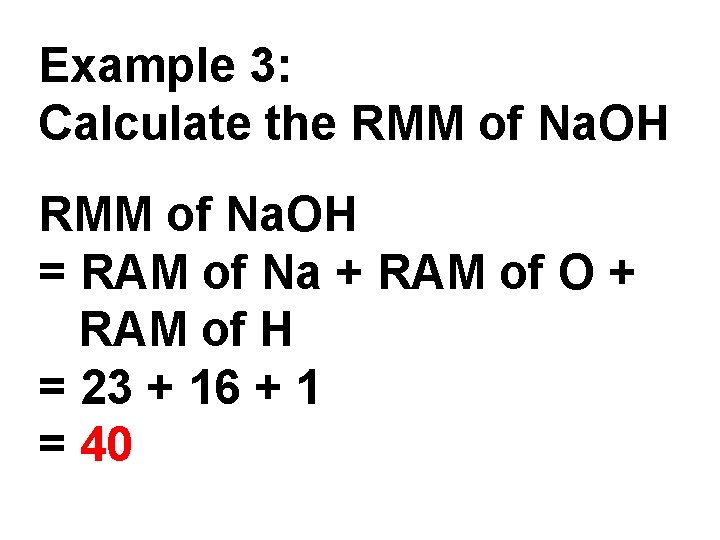
Example 3: Calculate the RMM of Na. OH = RAM of Na + RAM of O + RAM of H = 23 + 16 + 1 = 40

REMINDER !!! RAM & RMM is a number without unit

HOMEWORK: Exercise book: Try This Out 3. 1 page 17 of Practical Textbook Submit: Thursday, 10/2/11