CHAPTER 3 CHEMICAL FORMULAE EQUATIONS The Mole The




- Slides: 15

CHAPTER 3: CHEMICAL FORMULAE & EQUATIONS The Mole & The Mass of Substances 4 S 1 Monday, 14/2/2011 10. 45 – 11. 55 am

Previously…

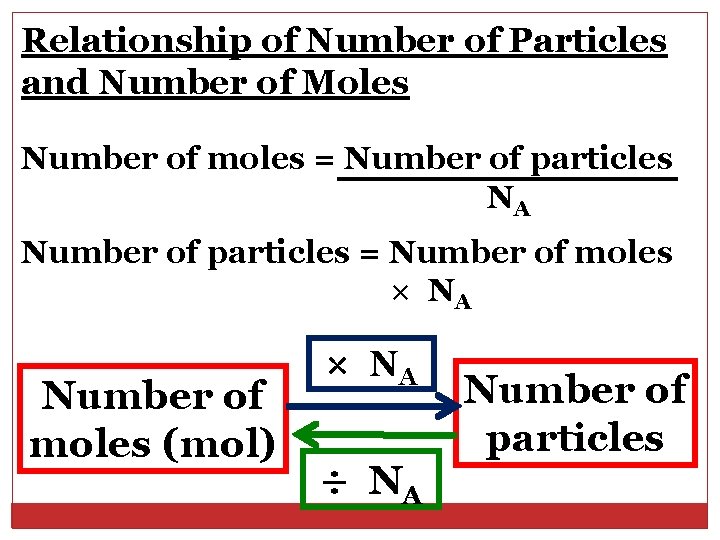
Relationship of Number of Particles and Number of Moles Number of moles = Number of particles NA Number of particles = Number of moles × NA Number of moles (mol) × NA ÷ NA Number of particles

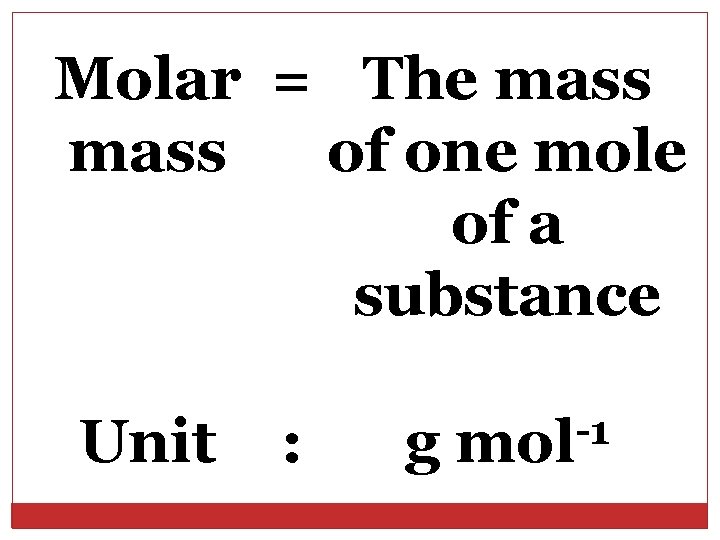
Molar = The mass of one mole of a substance Unit : g -1 mol

One mole of any substance contains 23 6. 02 x 10 particles. Therefore, the molar mass of a substance 23 contains 6. 02 x 10 particles of the substance

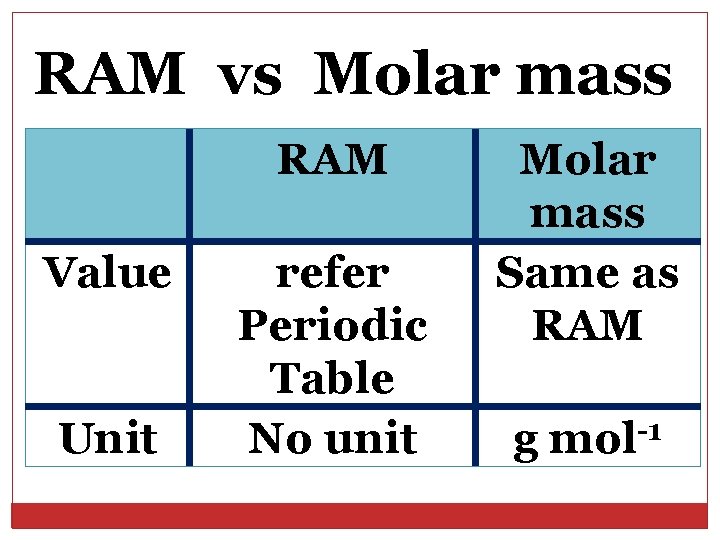
RAM vs Molar mass RAM Value Unit refer Periodic Table No unit Molar mass Same as RAM g -1 mol

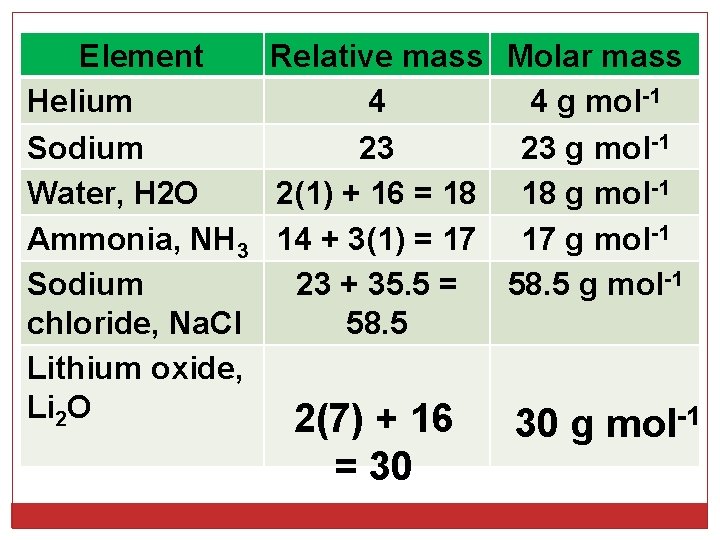
Element Relative mass Molar mass Helium 4 4 g mol-1 Sodium 23 23 g mol-1 Water, H 2 O 2(1) + 16 = 18 18 g mol-1 Ammonia, NH 3 14 + 3(1) = 17 17 g mol-1 Sodium 23 + 35. 5 = 58. 5 g mol-1 chloride, Na. Cl 58. 5 Lithium oxide, Li 2 O -1 2(7) + 16 = 30 30 g mol

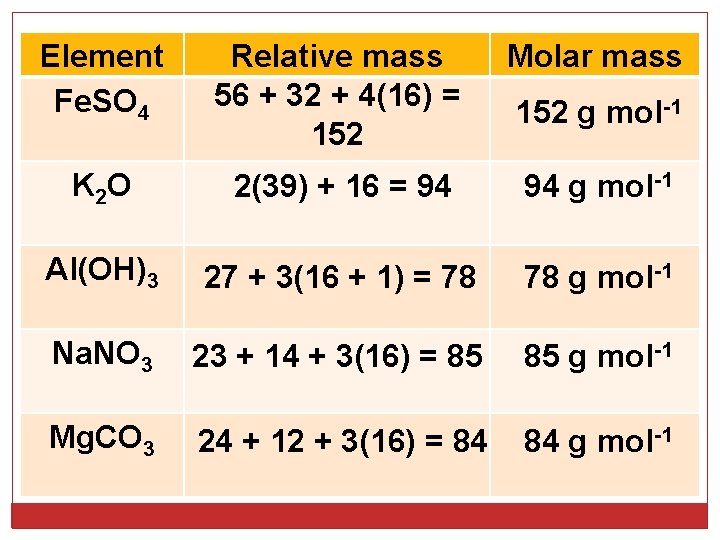
Element Fe. SO 4 Relative mass 56 + 32 + 4(16) = 152 Molar mass K 2 O 2(39) + 16 = 94 94 g mol-1 Al(OH)3 27 + 3(16 + 1) = 78 78 g mol-1 Na. NO 3 23 + 14 + 3(16) = 85 85 g mol-1 Mg. CO 3 24 + 12 + 3(16) = 84 84 g mol-1 152 g mol-1

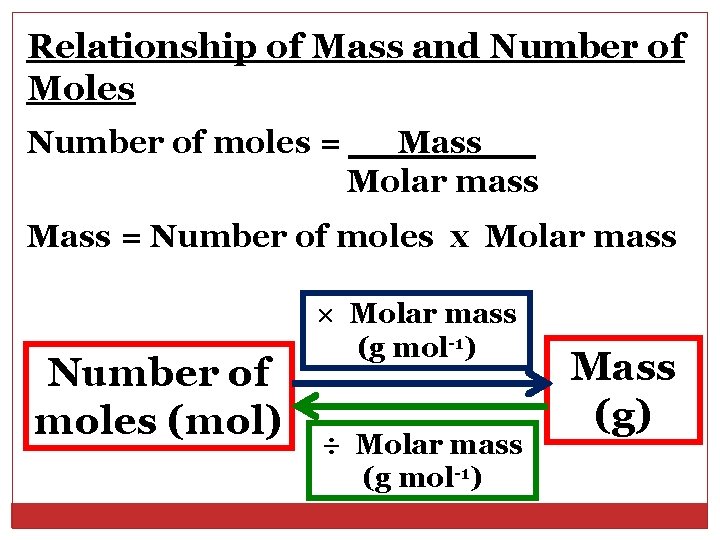
Relationship of Mass and Number of Moles Number of moles = Mass Molar mass Mass = Number of moles x Molar mass Number of moles (mol) × Molar mass (g mol-1) ÷ Molar mass (g mol-1) Mass (g)

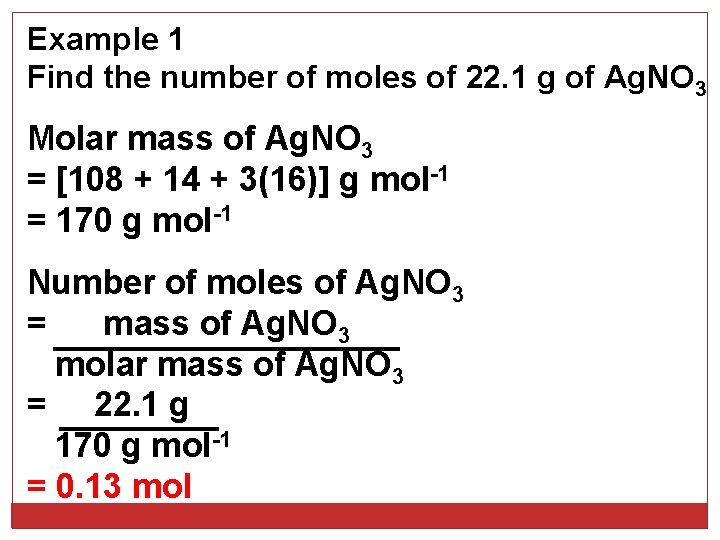
Example 1 Find the number of moles of 22. 1 g of Ag. NO 3 Molar mass of Ag. NO 3 = [108 + 14 + 3(16)] g mol-1 = 170 g mol-1 Number of moles of Ag. NO 3 = mass of Ag. NO 3 molar mass of Ag. NO 3 = 22. 1 g 170 g mol-1 = 0. 13 mol

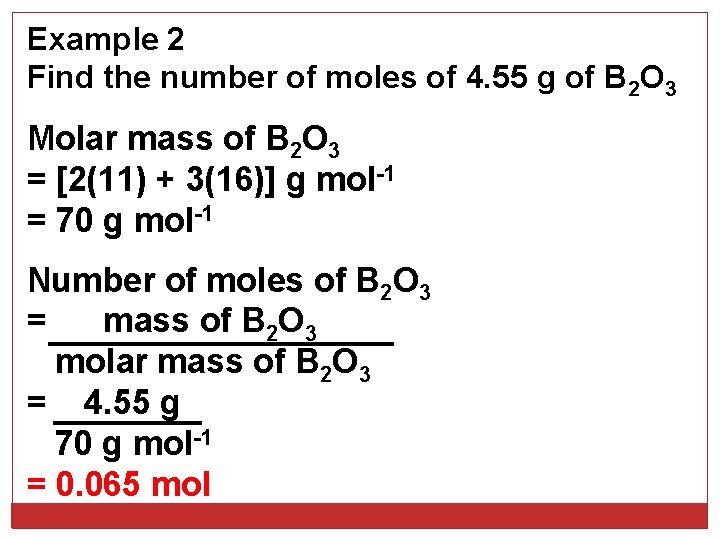
Example 2 Find the number of moles of 4. 55 g of B 2 O 3 Molar mass of B 2 O 3 = [2(11) + 3(16)] g mol-1 = 70 g mol-1 Number of moles of B 2 O 3 = mass of B 2 O 3 molar mass of B 2 O 3 = 4. 55 g 70 g mol-1 = 0. 065 mol

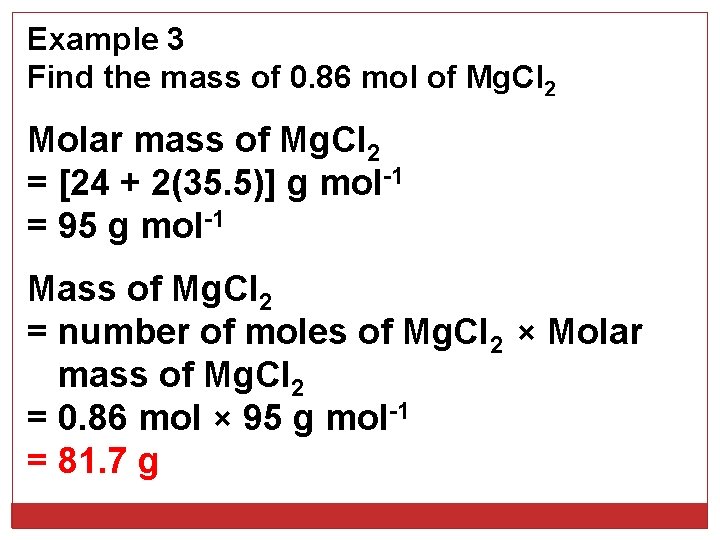
Example 3 Find the mass of 0. 86 mol of Mg. Cl 2 Molar mass of Mg. Cl 2 = [24 + 2(35. 5)] g mol-1 = 95 g mol-1 Mass of Mg. Cl 2 = number of moles of Mg. Cl 2 × Molar mass of Mg. Cl 2 = 0. 86 mol × 95 g mol-1 = 81. 7 g

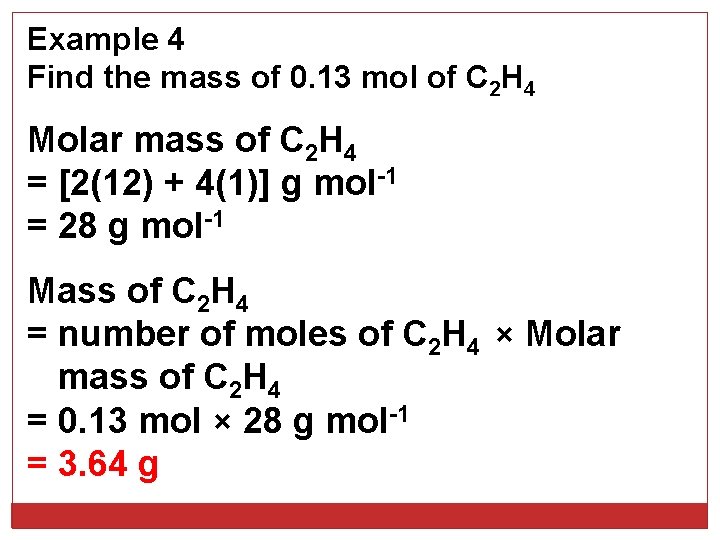
Example 4 Find the mass of 0. 13 mol of C 2 H 4 Molar mass of C 2 H 4 = [2(12) + 4(1)] g mol-1 = 28 g mol-1 Mass of C 2 H 4 = number of moles of C 2 H 4 × Molar mass of C 2 H 4 = 0. 13 mol × 28 g mol-1 = 3. 64 g

Exercise 1 Find the number of moles of 23 g of NO 2 Molar mass of NO 2 = [14 + 2(16)] g mol-1 = 46 g mol-1 Number of moles of NO 2 = mass of NO 2 molar mass of NO 2 = 23 g 46 g mol-1 = 0. 5 mol

Exercise 2 Find the mass of 0. 1 mol of SO 3 Molar mass of SO 3 = [32 + 3(16)] g mol-1 = 80 g mol-1 Mass of SO 3 = number of moles of SO 3 × Molar mass of SO 3 = 0. 1 mol × 80 g mol-1 = 8. 00 g