CHAPTER 3 CHEMICAL FORMULAE EQUATIONS The Mole The




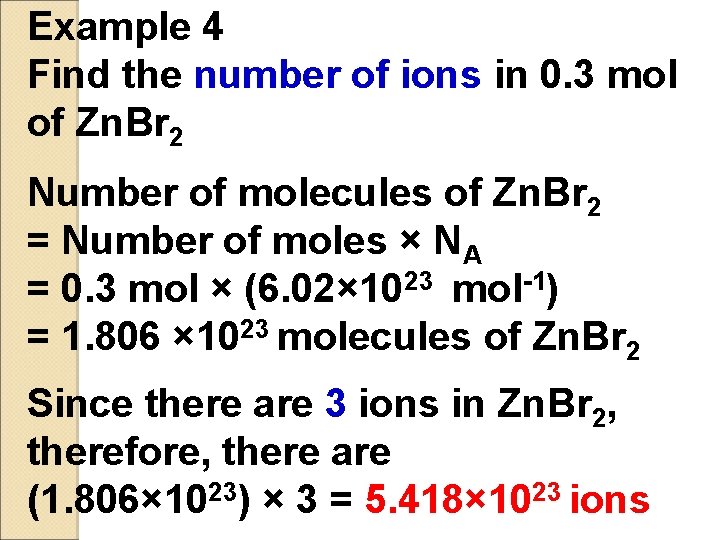
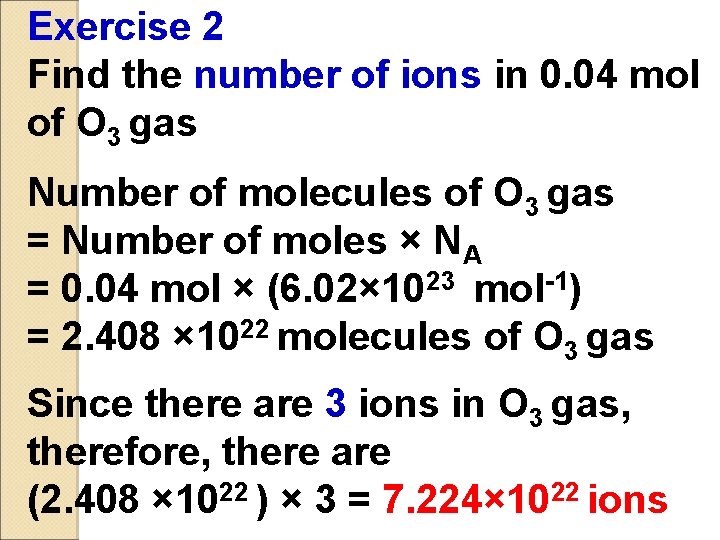
- Slides: 12

CHAPTER 3: CHEMICAL FORMULAE & EQUATIONS The Mole & The Number of Particles 4 S 1 Thursday, 10/2/11 7. 30 – 8. 40 am

A mole is an amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12

1 pair = 2 items 1 dozen = 12 items 23 1 mole = 6. 02 x 10 items

23 10 The value 6. 02 x is called the Avogadro constant ? The number of particles in one mole of a substance

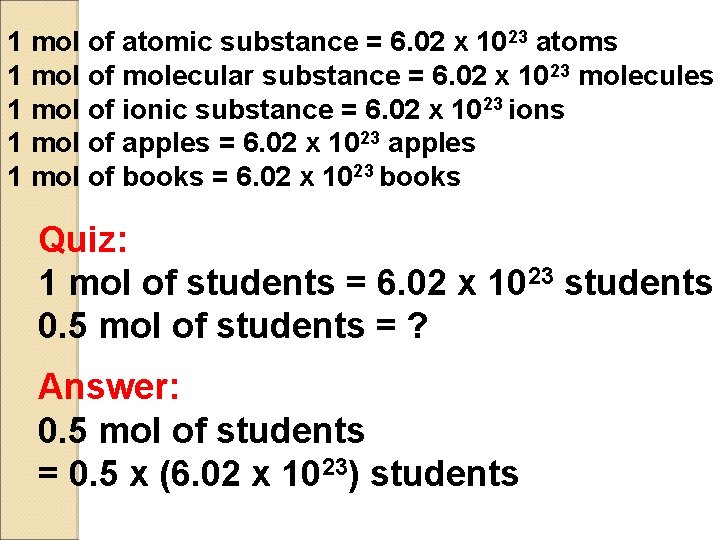
1 mol of atomic substance = 6. 02 x 1023 atoms 1 mol of molecular substance = 6. 02 x 1023 molecules 1 mol of ionic substance = 6. 02 x 1023 ions 1 mol of apples = 6. 02 x 1023 apples 1 mol of books = 6. 02 x 1023 books Quiz: 1 mol of students = 6. 02 x 1023 students 0. 5 mol of students = ? Answer: 0. 5 mol of students = 0. 5 x (6. 02 x 1023) students

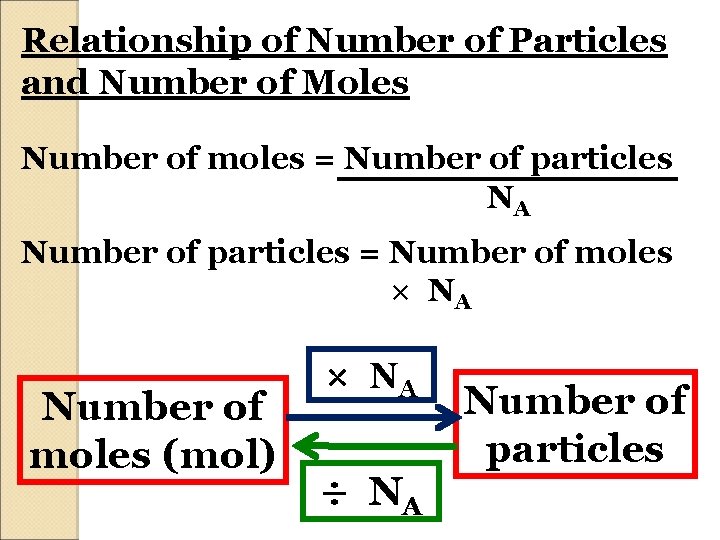
Relationship of Number of Particles and Number of Moles Number of moles = Number of particles NA Number of particles = Number of moles × NA Number of moles (mol) × NA ÷ NA Number of particles

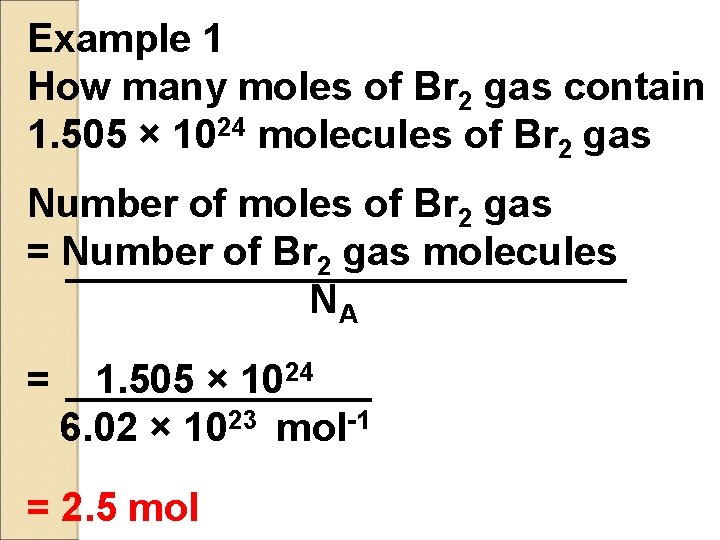
Example 1 How many moles of Br 2 gas contain 1. 505 × 1024 molecules of Br 2 gas Number of moles of Br 2 gas = Number of Br 2 gas molecules NA = 1. 505 × 1024 6. 02 × 1023 mol-1 = 2. 5 mol

Example 2 How many moles of water are there in 3. 01 × 1022 water molecules Number of moles of water molecules = Number of water molecules NA = 3. 01 × 1022 6. 02 × 1023 mol-1 = 0. 05 mol

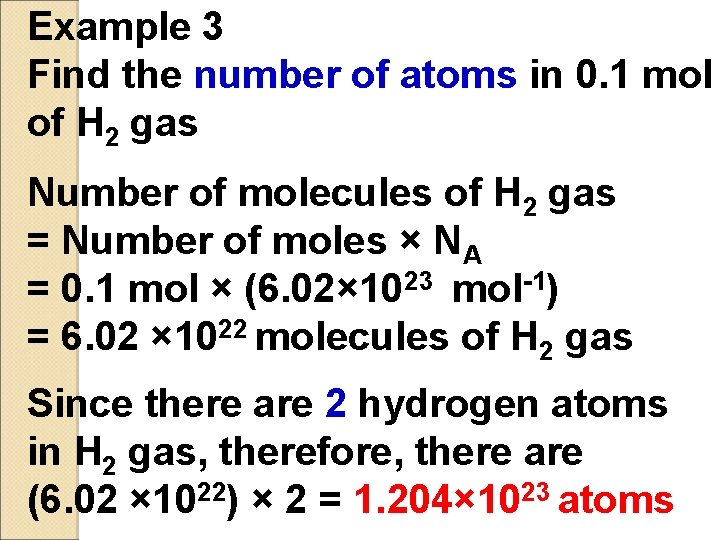
Example 3 Find the number of atoms in 0. 1 mol of H 2 gas Number of molecules of H 2 gas = Number of moles × NA = 0. 1 mol × (6. 02× 1023 mol-1) = 6. 02 × 1022 molecules of H 2 gas Since there are 2 hydrogen atoms in H 2 gas, therefore, there are (6. 02 × 1022) × 2 = 1. 204× 1023 atoms
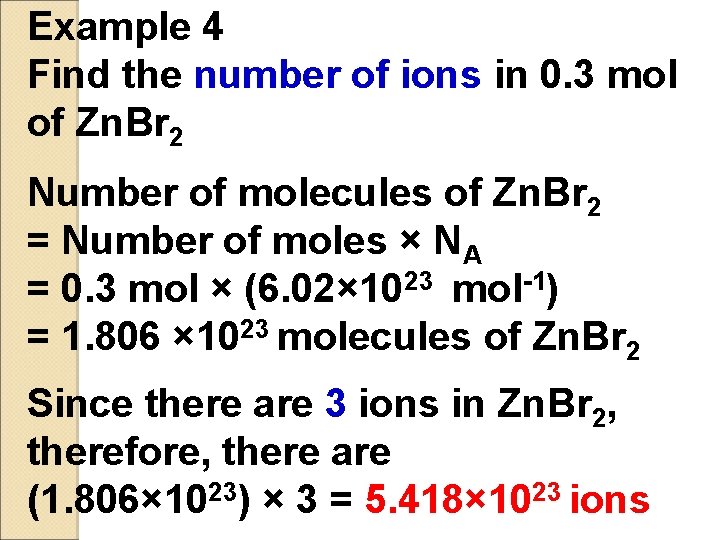
Example 4 Find the number of ions in 0. 3 mol of Zn. Br 2 Number of molecules of Zn. Br 2 = Number of moles × NA = 0. 3 mol × (6. 02× 1023 mol-1) = 1. 806 × 1023 molecules of Zn. Br 2 Since there are 3 ions in Zn. Br 2, therefore, there are (1. 806× 1023) × 3 = 5. 418× 1023 ions

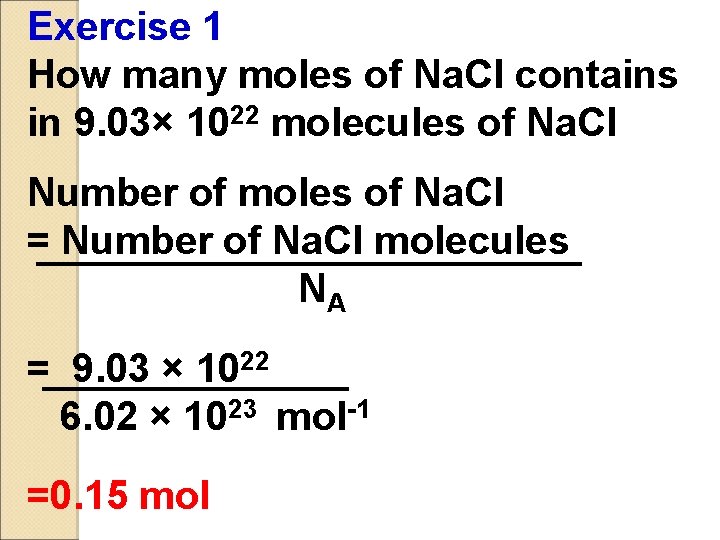
Exercise 1 How many moles of Na. Cl contains in 9. 03× 1022 molecules of Na. Cl Number of moles of Na. Cl = Number of Na. Cl molecules NA = 9. 03 × 1022 6. 02 × 1023 mol-1 =0. 15 mol
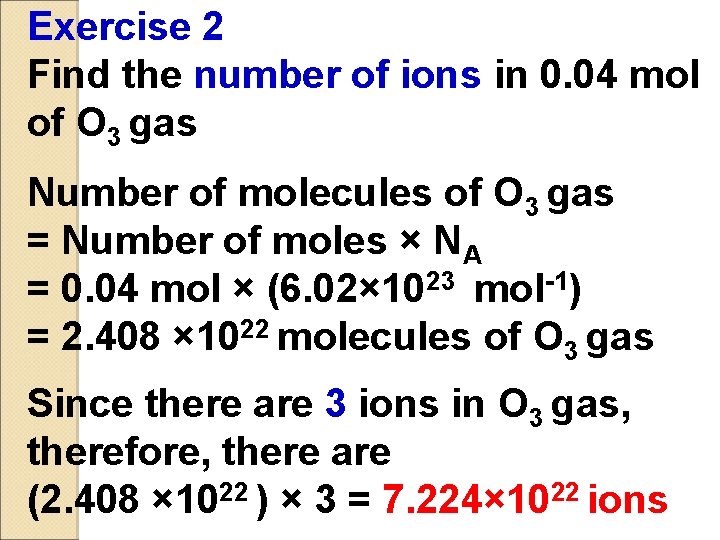
Exercise 2 Find the number of ions in 0. 04 mol of O 3 gas Number of molecules of O 3 gas = Number of moles × NA = 0. 04 mol × (6. 02× 1023 mol-1) = 2. 408 × 1022 molecules of O 3 gas Since there are 3 ions in O 3 gas, therefore, there are (2. 408 × 1022 ) × 3 = 7. 224× 1022 ions