Titration p H curves 17 3 Titration A
![Titration & p. H curves [17. 3] Titration & p. H curves [17. 3]](https://slidetodoc.com/presentation_image_h2/6368bdc2f3dd93f3547aa73e97942e08/image-1.jpg)

![� 1. 8 x 10 -5 = x 2 0. 200 x = [H+] � 1. 8 x 10 -5 = x 2 0. 200 x = [H+]](https://slidetodoc.com/presentation_image_h2/6368bdc2f3dd93f3547aa73e97942e08/image-11.jpg)

- Slides: 21
![Titration p H curves 17 3 Titration & p. H curves [17. 3]](https://slidetodoc.com/presentation_image_h2/6368bdc2f3dd93f3547aa73e97942e08/image-1.jpg)
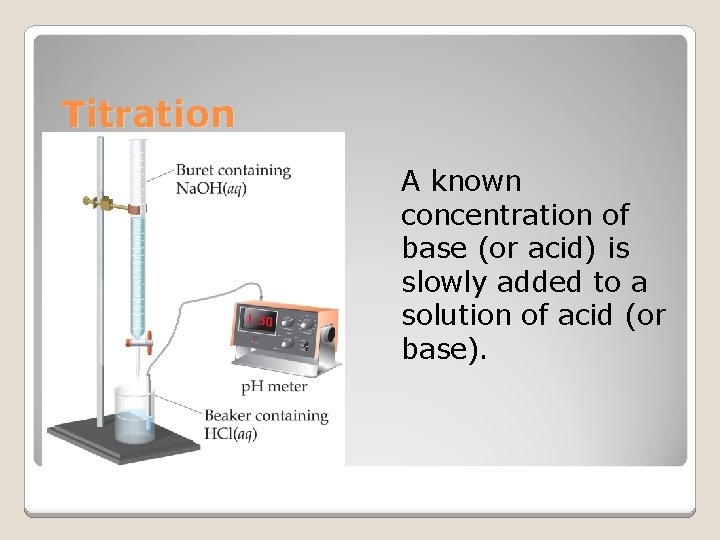
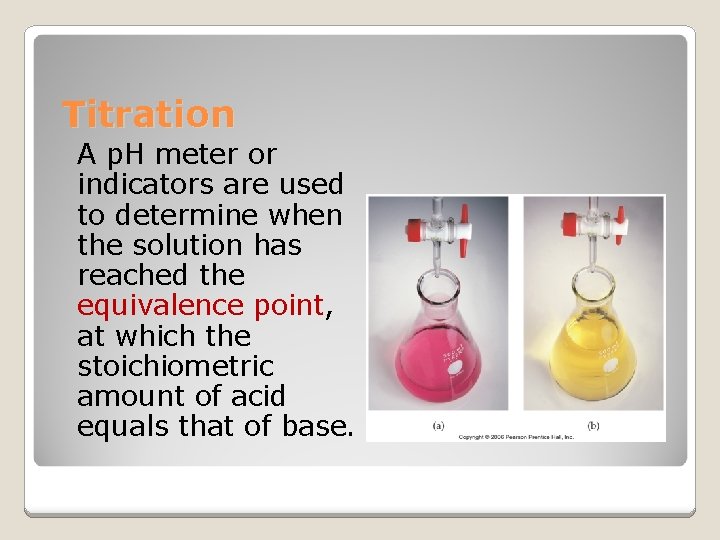
Titration & p. H curves [17. 3]

Titration A known concentration of base (or acid) is slowly added to a solution of acid (or base).

Titration A p. H meter or indicators are used to determine when the solution has reached the equivalence point, at which the stoichiometric amount of acid equals that of base.

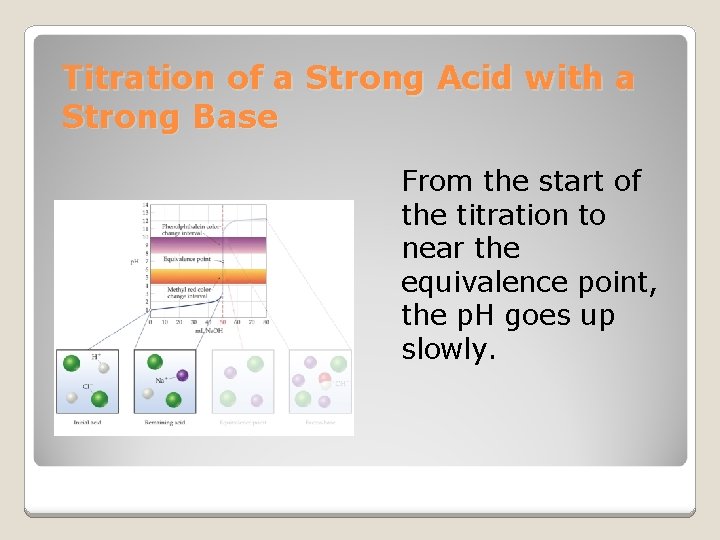
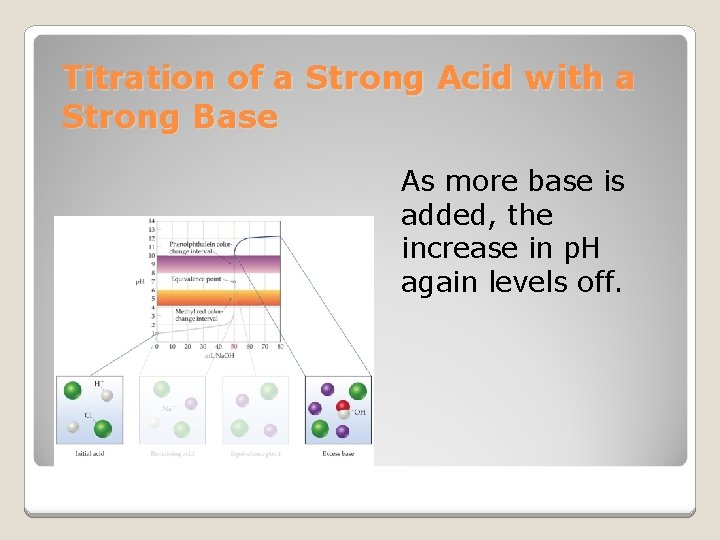
Titration of a Strong Acid with a Strong Base From the start of the titration to near the equivalence point, the p. H goes up slowly.

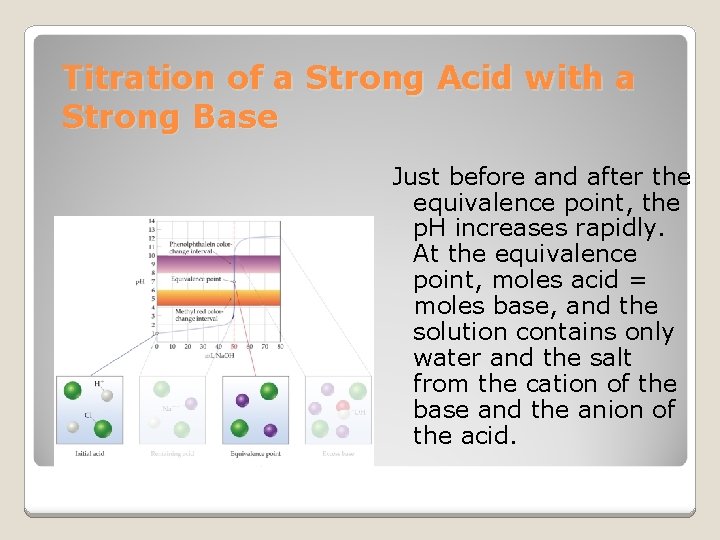
Titration of a Strong Acid with a Strong Base Just before and after the equivalence point, the p. H increases rapidly. At the equivalence point, moles acid = moles base, and the solution contains only water and the salt from the cation of the base and the anion of the acid.

Titration of a Strong Acid with a Strong Base As more base is added, the increase in p. H again levels off.

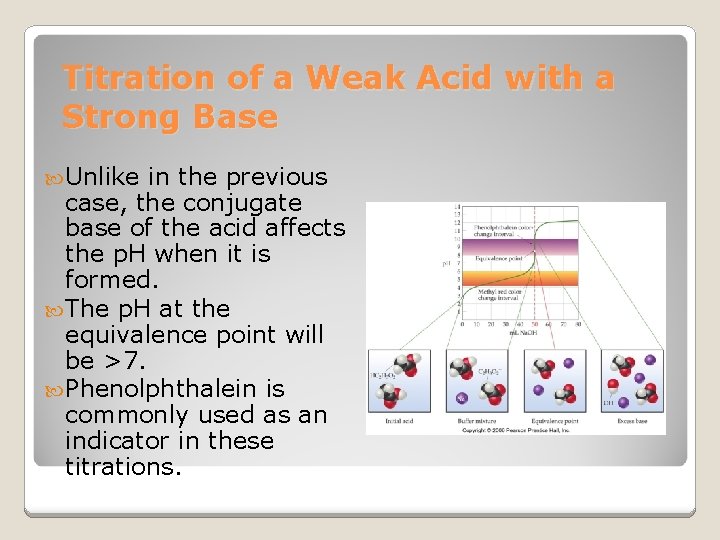
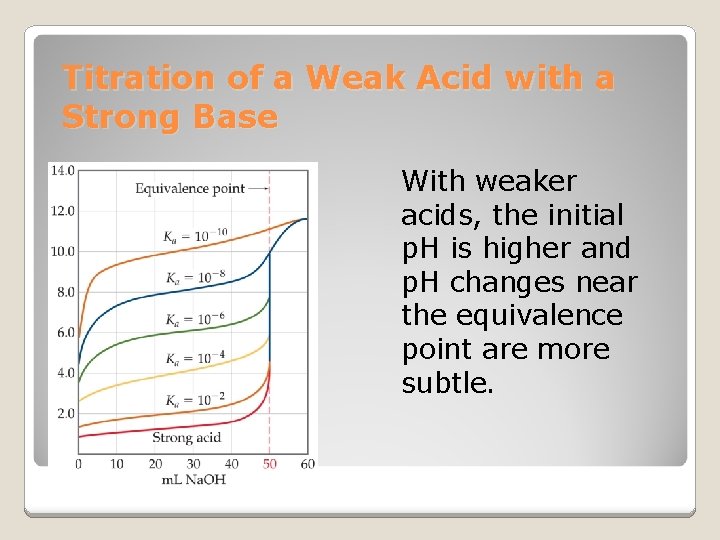
Titration of a Weak Acid with a Strong Base Unlike in the previous case, the conjugate base of the acid affects the p. H when it is formed. The p. H at the equivalence point will be >7. Phenolphthalein is commonly used as an indicator in these titrations.

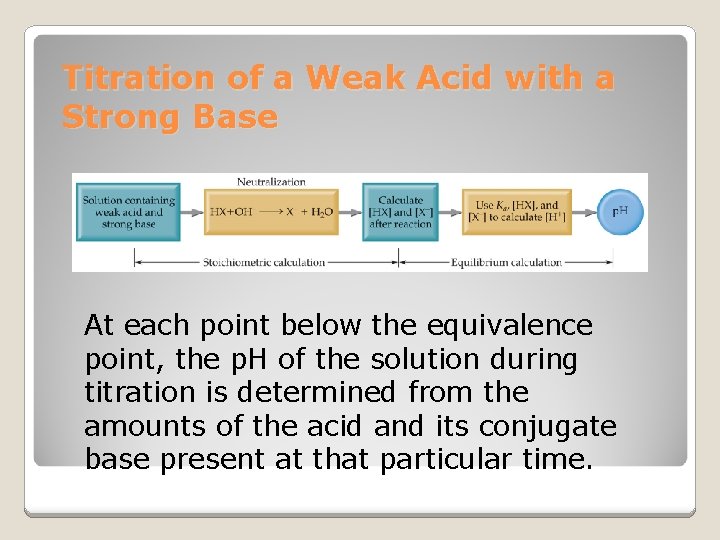
Titration of a Weak Acid with a Strong Base At each point below the equivalence point, the p. H of the solution during titration is determined from the amounts of the acid and its conjugate base present at that particular time.

Zumdahl #59 Titration Problem Solving

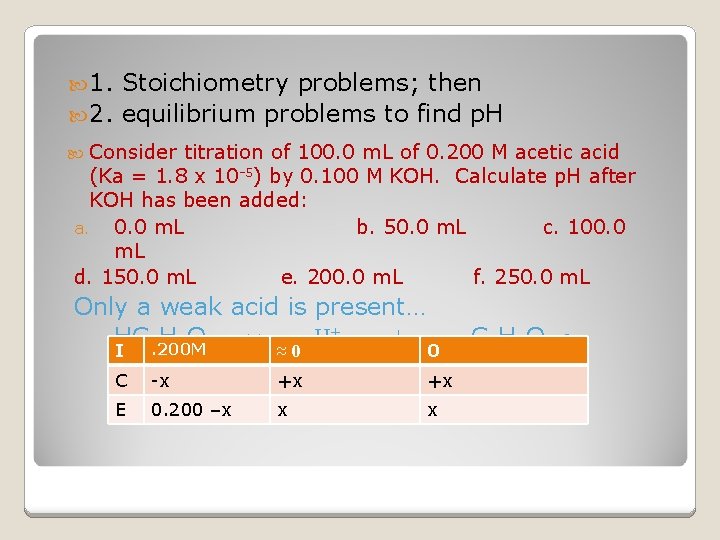
1. Stoichiometry problems; then 2. equilibrium problems to find p. H Consider titration of 100. 0 m. L of 0. 200 M acetic acid (Ka = 1. 8 x 10 -5) by 0. 100 M KOH. Calculate p. H after KOH has been added: a. 0. 0 m. L b. 50. 0 m. L c. 100. 0 m. L d. 150. 0 m. L e. 200. 0 m. L f. 250. 0 m. L Only a weak acid is present… HC 2. 200 M H 3 O 2 ↔ ≈ 0 H + + I 0 C -x +x +x E 0. 200 –x x x C 2 H 3 O 2 -
![1 8 x 10 5 x 2 0 200 x H � 1. 8 x 10 -5 = x 2 0. 200 x = [H+]](https://slidetodoc.com/presentation_image_h2/6368bdc2f3dd93f3547aa73e97942e08/image-11.jpg)
� 1. 8 x 10 -5 = x 2 0. 200 x = [H+] = 1. 9 x 10 -3 M p. H = 2. 72 b. Added OH- will react completely with the best acid present: HC 2 H 3 O 2 + OH↔ C 2 H 3 O 2 - + H 2 O I . 200 M x. 1000 L = o. 0200 moles 0. 100 M x 0. 050 L = 0. 00500 moles 0 C -0. 00500 +0. 00500 E 0. 0150 mols 0 0. 00500 mols ----

�After reaction we have a buffer solution � p. H = -log (1. 8 x 10 -5) + log (0. 00500/0. 150 L) (0. 0150 /0. 150 L) p. H = 4. 74 + (-0. 477) = 4. 26 c. HC 2 H 3 O 2 + OH↔ C 2 H 3 O 2 - + H 2 O I 0. 200 M x 0. 1000 L = 0. 0200 mols 0. 100 M x 0. 1000 L = 0. 0100 moles 0 C -0. 0100 +0. 0100 E 0. 0100 mols 0 0. 0100 mols p. H = -log (1. 8 x 10 -5) + log (0. 0100/0. 200 L) (0. 0100 /0. 200 L) p. H = 4. 74

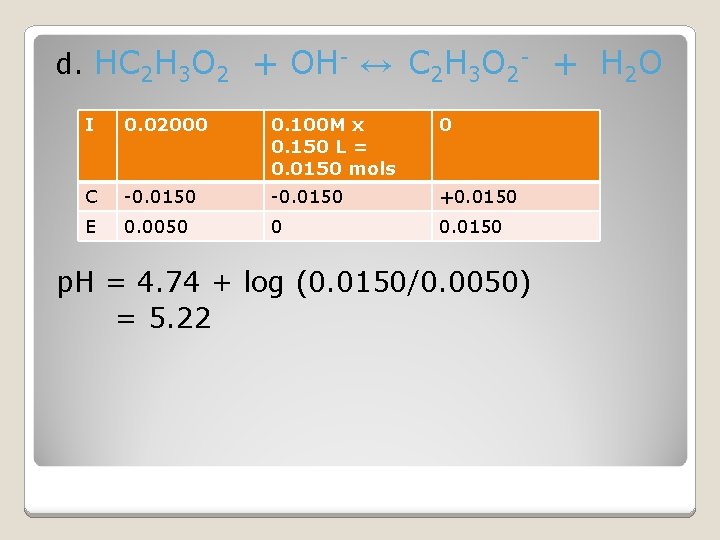
d. HC 2 H 3 O 2 + OH- ↔ C 2 H 3 O 2 - + H 2 O I 0. 02000 0. 100 M x 0. 150 L = 0. 0150 mols 0 C -0. 0150 +0. 0150 E 0. 0050 0 0. 0150 p. H = 4. 74 + log (0. 0150/0. 0050) = 5. 22

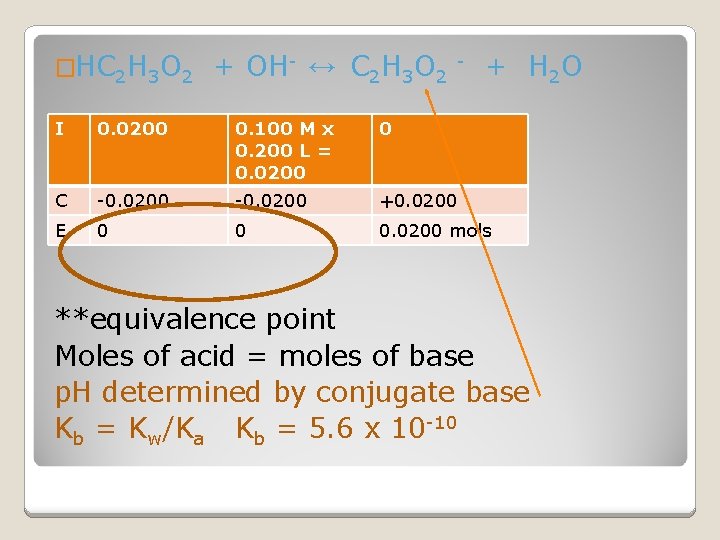
�HC 2 H 3 O 2 + OH- ↔ C 2 H 3 O 2 - + H 2 O I 0. 0200 0. 100 M x 0. 200 L = 0. 0200 0 C -0. 0200 +0. 0200 E 0 0 0. 0200 mols **equivalence point Moles of acid = moles of base p. H determined by conjugate base Kb = Kw/Ka Kb = 5. 6 x 10 -10

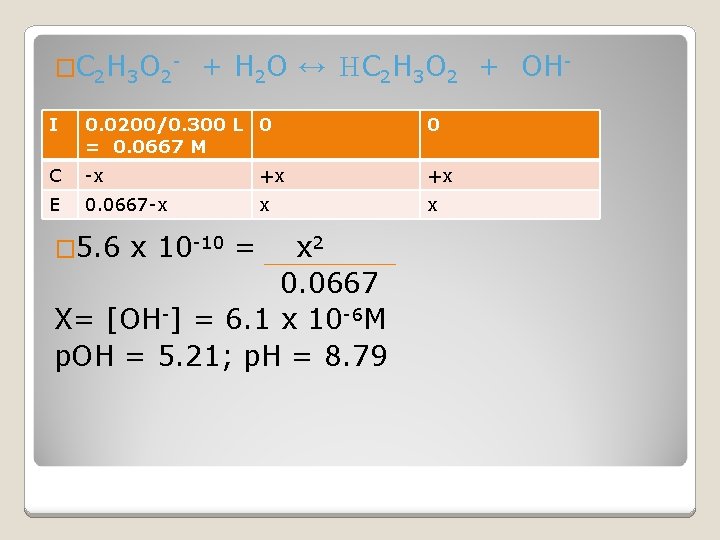
�C 2 H 3 O 2 - + H 2 O ↔ HC 2 H 3 O 2 + OH- I 0. 0200/0. 300 L 0 = 0. 0667 M 0 C -x +x +x E 0. 0667 -x x x � 5. 6 x 10 -10 = x 2 0. 0667 X= [OH-] = 6. 1 x 10 -6 M p. OH = 5. 21; p. H = 8. 79

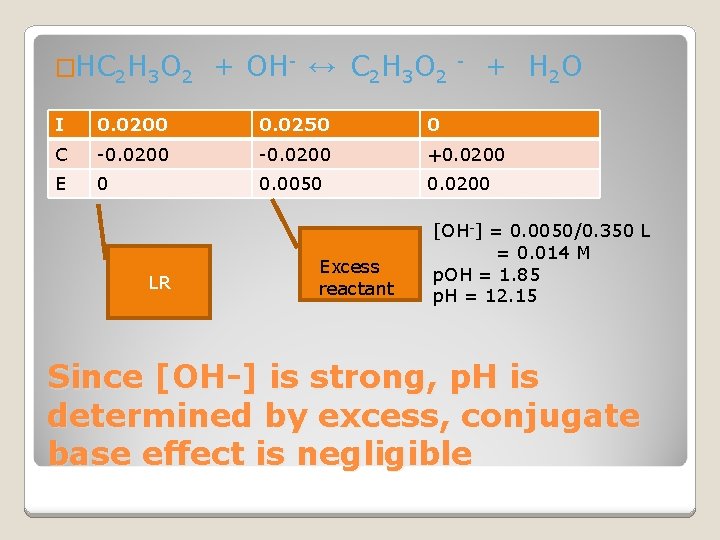
�HC 2 H 3 O 2 + OH- ↔ C 2 H 3 O 2 - + H 2 O I 0. 0200 0. 0250 0 C -0. 0200 +0. 0200 E 0 0. 0050 0. 0200 LR Excess reactant [OH-] = 0. 0050/0. 350 L = 0. 014 M p. OH = 1. 85 p. H = 12. 15 Since [OH-] is strong, p. H is determined by excess, conjugate base effect is negligible

Titration of a Weak Acid with a Strong Base With weaker acids, the initial p. H is higher and p. H changes near the equivalence point are more subtle.

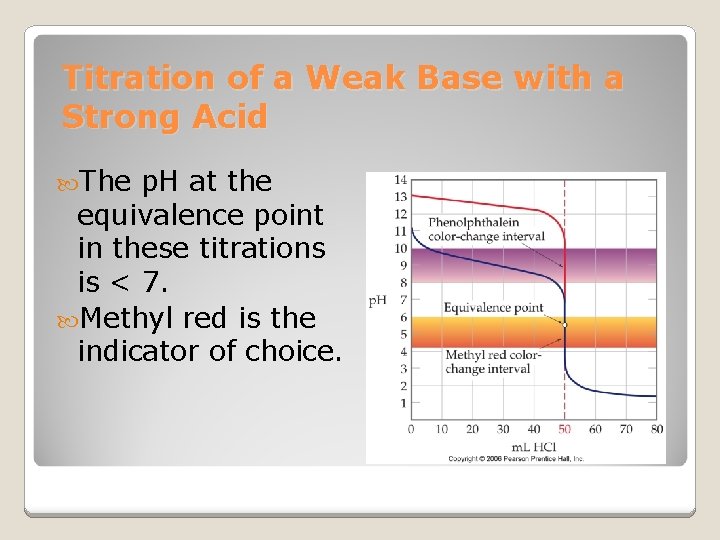
Titration of a Weak Base with a Strong Acid The p. H at the equivalence point in these titrations is < 7. Methyl red is the indicator of choice.

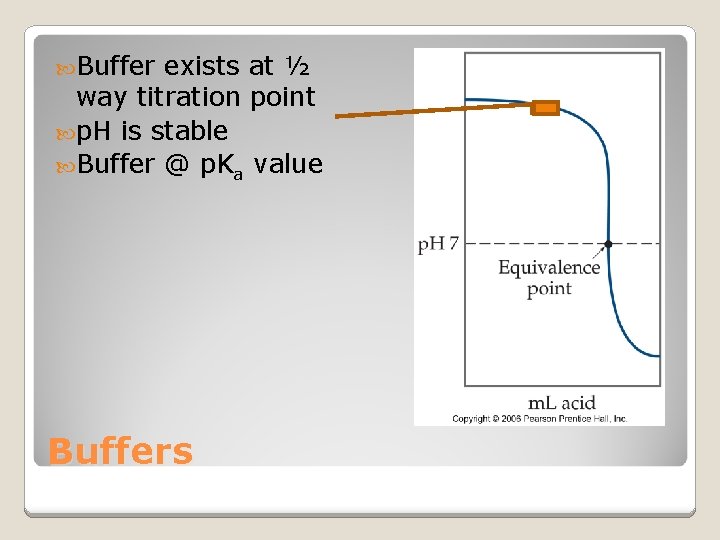
Buffer exists at ½ way titration point p. H is stable Buffer @ p. Ka value Buffers

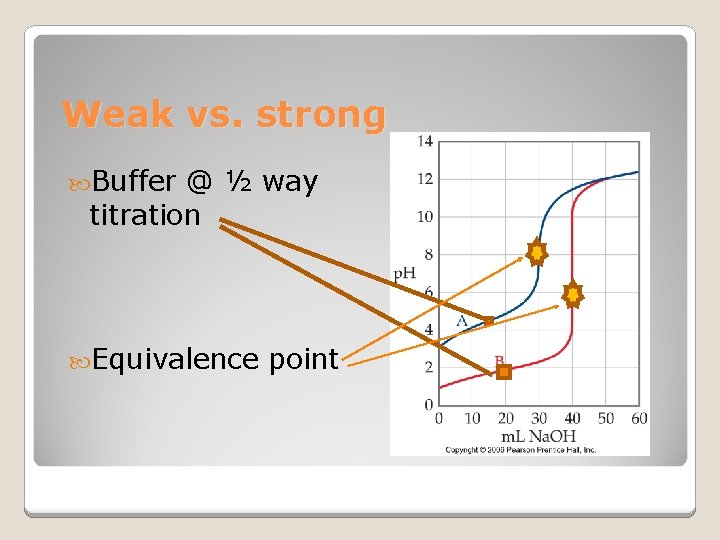
Weak vs. strong Buffer @ ½ way titration Equivalence point

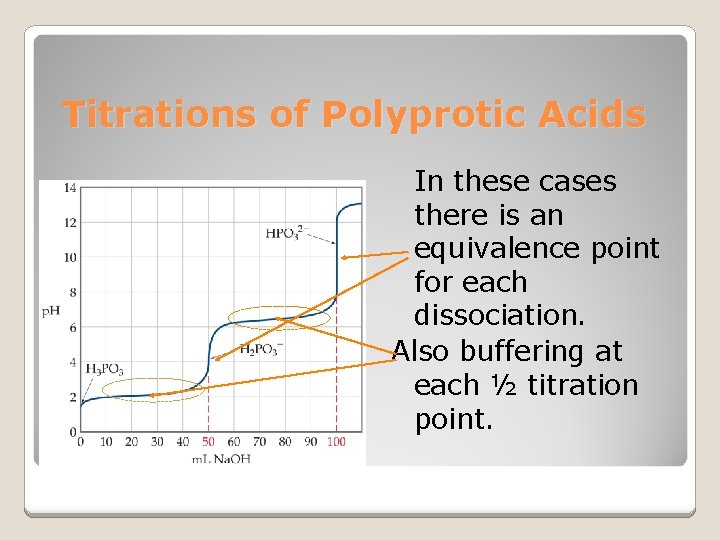
Titrations of Polyprotic Acids In these cases there is an equivalence point for each dissociation. Also buffering at each ½ titration point.