1 Titration of Weak acids Titration of a

![Titration of a Weak Acid Continue -] p. K + log [A p. H Titration of a Weak Acid Continue -] p. K + log [A p. H](https://slidetodoc.com/presentation_image/3a35582bf8afdd20b99fef999c46d8ef/image-7.jpg)
![Titration of a Weak Acid Continue -] p. K + log [A p. H Titration of a Weak Acid Continue -] p. K + log [A p. H](https://slidetodoc.com/presentation_image/3a35582bf8afdd20b99fef999c46d8ef/image-8.jpg)
![Titration of a Weak Acid Continue [A-] = 0. 05/1 = 0. 05 M Titration of a Weak Acid Continue [A-] = 0. 05/1 = 0. 05 M](https://slidetodoc.com/presentation_image/3a35582bf8afdd20b99fef999c46d8ef/image-9.jpg)
- Slides: 12

1. Titration of Weak acids

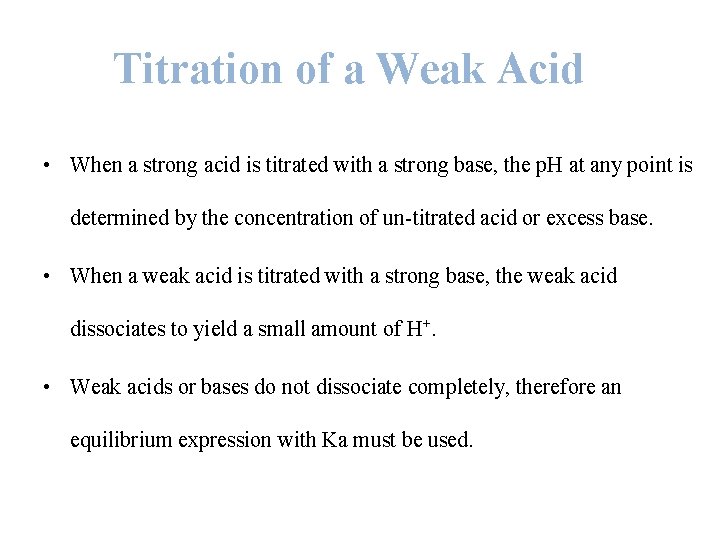
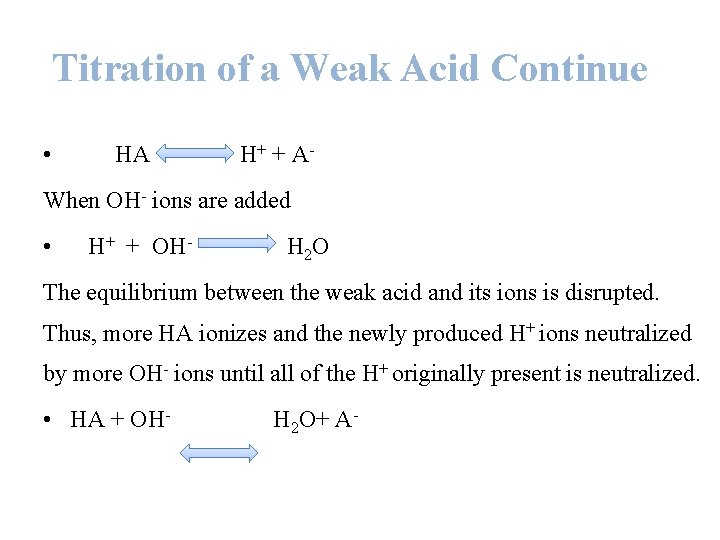
Titration of a Weak Acid • When a strong acid is titrated with a strong base, the p. H at any point is determined by the concentration of un-titrated acid or excess base. • When a weak acid is titrated with a strong base, the weak acid dissociates to yield a small amount of H+. • Weak acids or bases do not dissociate completely, therefore an equilibrium expression with Ka must be used.

Titration of a Weak Acid Continue • HA H+ + A- When OH- ions are added • H+ + OH- H 2 O The equilibrium between the weak acid and its ions is disrupted. Thus, more HA ionizes and the newly produced H+ ions neutralized by more OH- ions until all of the H+ originally present is neutralized. • HA + OH- H 2 O+ A-

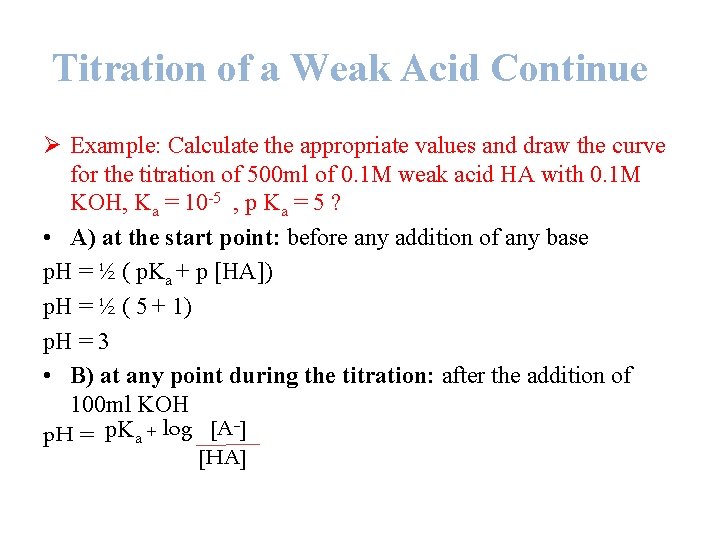
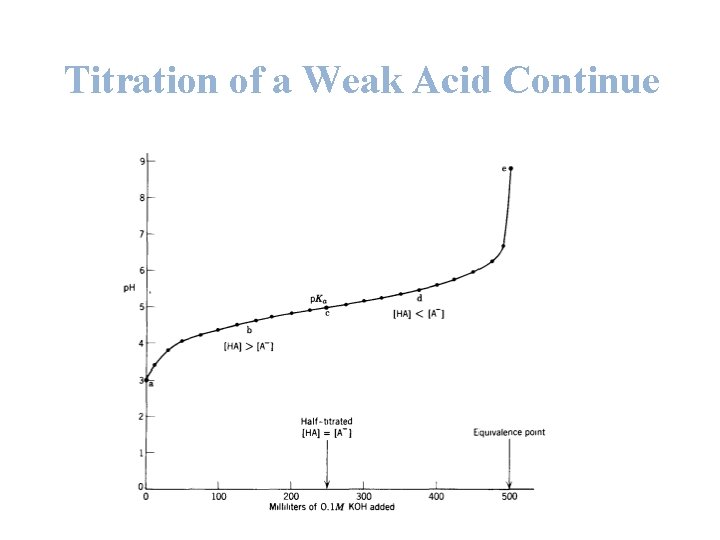
Titration of a Weak Acid Continue Ø Example: Calculate the appropriate values and draw the curve for the titration of 500 ml of 0. 1 M weak acid HA with 0. 1 M KOH, Ka = 10 -5 , p Ka = 5 ? • A) at the start point: before any addition of any base p. H = ½ ( p. Ka + p [HA]) p. H = ½ ( 5 + 1) p. H = 3 • B) at any point during the titration: after the addition of 100 ml KOH p. H = p. Ka + log [A ] [HA]

Titration of a Weak Acid Continue Since KOH + HA KA + H 2 O No. of moles of KOH added = M * V = 0. 1 * 0. 1 = 0. 01 mole No. of moles of original HA= M * V = 0. 1 * 0. 5 = 0. 05 mole 1 mole of OH- will react with 1 mole of HA to produces 1 mole of salt. Thus, the no. of moles of salt produced = 0. 01 mole. No. of moles of remaining HA added=moles of HA originally present – moles of HA titrated to salt. p. H = p. Ka + log [A ] [HA] = 0. 05 – 0. 01 = 0. 04 mole

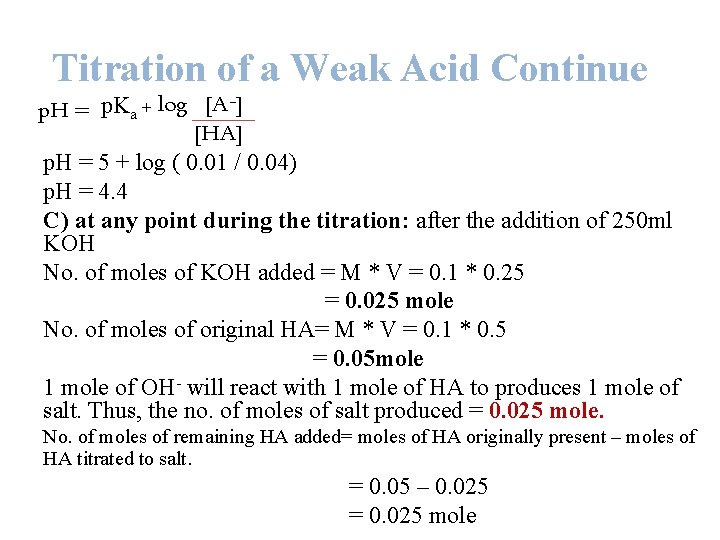
Titration of a Weak Acid Continue p. H = p. Ka + log [A ] [HA] p. H = 5 + log ( 0. 01 / 0. 04) p. H = 4. 4 C) at any point during the titration: after the addition of 250 ml KOH No. of moles of KOH added = M * V = 0. 1 * 0. 25 = 0. 025 mole No. of moles of original HA= M * V = 0. 1 * 0. 5 = 0. 05 mole 1 mole of OH- will react with 1 mole of HA to produces 1 mole of salt. Thus, the no. of moles of salt produced = 0. 025 mole. No. of moles of remaining HA added= moles of HA originally present – moles of HA titrated to salt. = 0. 05 – 0. 025 = 0. 025 mole
![Titration of a Weak Acid Continue p K log A p H Titration of a Weak Acid Continue -] p. K + log [A p. H](https://slidetodoc.com/presentation_image/3a35582bf8afdd20b99fef999c46d8ef/image-7.jpg)
Titration of a Weak Acid Continue -] p. K + log [A p. H = a [HA] p. H = 5 + log ( 0. 025 / 0. 025) p. H = 5 Here the [A-] = [HA] thus, p. H = p. Ka D) at any point during the titration: after the addition of 375 ml KOH No. of moles of KOH added = M * V = 0. 1 * 0. 375 = 0. 0375 mole No. of moles of original HA= M * V = 0. 1 * 0. 5 = 0. 05 mole 1 mole of OH- will react with 1 mole of HA to produces 1 mole of salt. Thus, the no. of moles of salt produced = 0. 0375 mole. No. of moles of remaining HA added= moles of HA originally present – moles of HA titrated to salt. = 0. 05 – 0. 0375 = 0. 0125 mole
![Titration of a Weak Acid Continue p K log A p H Titration of a Weak Acid Continue -] p. K + log [A p. H](https://slidetodoc.com/presentation_image/3a35582bf8afdd20b99fef999c46d8ef/image-8.jpg)
Titration of a Weak Acid Continue -] p. K + log [A p. H = a [HA] p. H = 5 + log ( 0. 0375/ 0. 0125) p. H = 5. 48 E) at the end point of the titration: after the addition of 500 ml KOH No. of moles of KOH added = M * V = 0. 1 * 0. 5 = 0. 05 mole No. of moles of original HA= M * V = 0. 1 * 0. 5 = 0. 05 mole 1 mole of OH- will react with 1 mole of HA to produces 1 mole of salt. Thus, the no. of moles of salt produced = 0. 05 mole. The final volume of the solution = 500+500 = 1000 ml
![Titration of a Weak Acid Continue A 0 051 0 05 M Titration of a Weak Acid Continue [A-] = 0. 05/1 = 0. 05 M](https://slidetodoc.com/presentation_image/3a35582bf8afdd20b99fef999c46d8ef/image-9.jpg)
Titration of a Weak Acid Continue [A-] = 0. 05/1 = 0. 05 M p [A-] = -log 0. 05 = 1. 3 p. OH = ½ ( p. Kb + p [A-]) = ½ ( 9 + 1. 3) = 5. 15 p. H= p. Kw – p. OH = 14 - 5. 15 =8. 85

Titration of a Weak Acid Continue

Titration of a Weak Acid Continue From the previous example: A) All HA is in the form of CH 3 COOH B) [CH 3 COOH] > [CH 3 COO-] C) [CH 3 COOH] = [CH 3 COO-] D) [CH 3 COOH] < [CH 3 COO-] E) All as CH 3 COO-

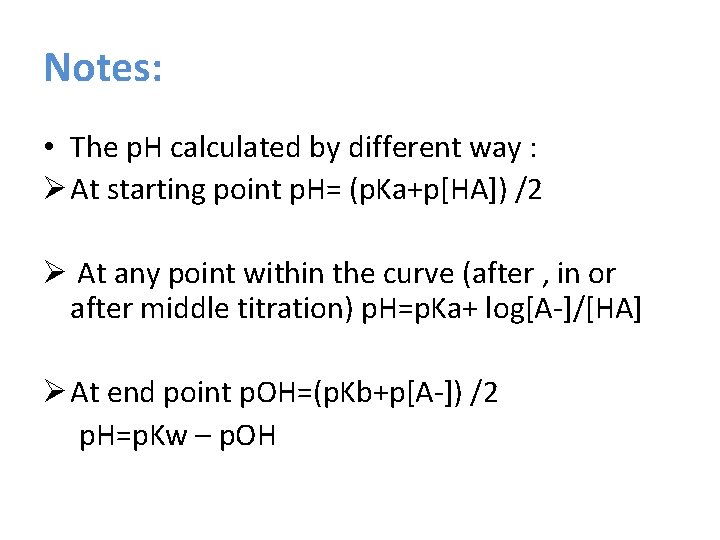
Notes: • The p. H calculated by different way : Ø At starting point p. H= (p. Ka+p[HA]) /2 Ø At any point within the curve (after , in or after middle titration) p. H=p. Ka+ log[A-]/[HA] Ø At end point p. OH=(p. Kb+p[A-]) /2 p. H=p. Kw – p. OH
 Strong acid weak base titration graph
Strong acid weak base titration graph Strong acids 7
Strong acids 7 Strong vs weak acids
Strong vs weak acids Acids and bases
Acids and bases Weak acid and weak base reaction
Weak acid and weak base reaction Strong acids
Strong acids Lysine titration
Lysine titration Titration curve for arginine
Titration curve for arginine Titration curve of amino acids
Titration curve of amino acids Titration plot
Titration plot Strong acid weak base titration
Strong acid weak base titration Strong acid titration curve
Strong acid titration curve How to read a titration curve
How to read a titration curve