Acids Lesson 20 Titration Curves Titration Curves A



- Slides: 47

Acids Lesson 20 Titration Curves

Titration Curves A titration curve is a graph of the p. H changes that occur during an acid -base titration versus the volume of acid or base added. There are three types of titration curves. You need to be able to recognize each and then choose a suitable indicator for that titration. The equivalence point is the end of a titration where the stoichiometry of the reaction is exactly satisfied, or moles H+ = moles OH-. The transition point refers to when an indicator changes color and [HInd] = [Ind-].

Choosing an Indicator When you choose an indicator, you must pick one so that the transition point of the indicator matches the equivalence point of the titration. When acid base titration is at the equivalence point, the acid has neutralized the base leaving only a salt and water. The p. H of the equivalence point depends on type of salt. Rule of thumb If the salt is neutral the equivalence point = 7 If the salt is basic the equivalence point = 9 If the salt is acidic the equivalence point = 5

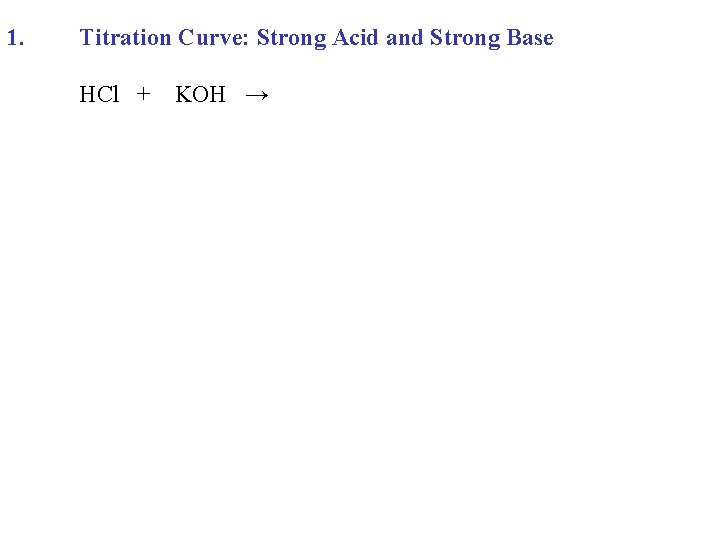
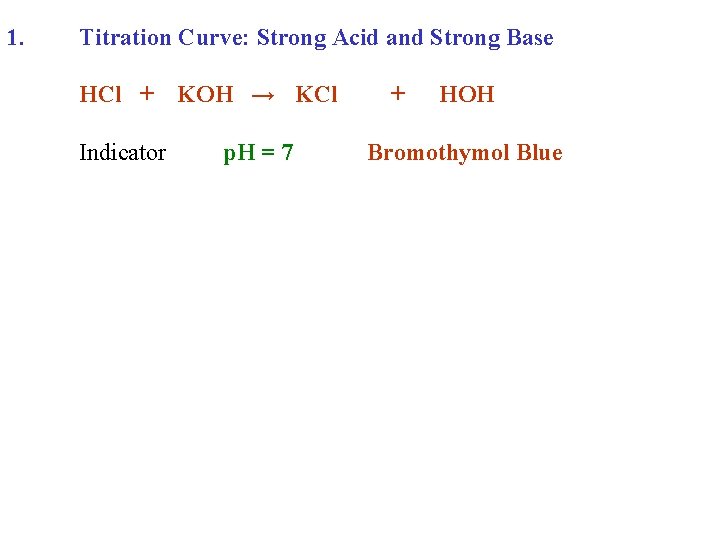
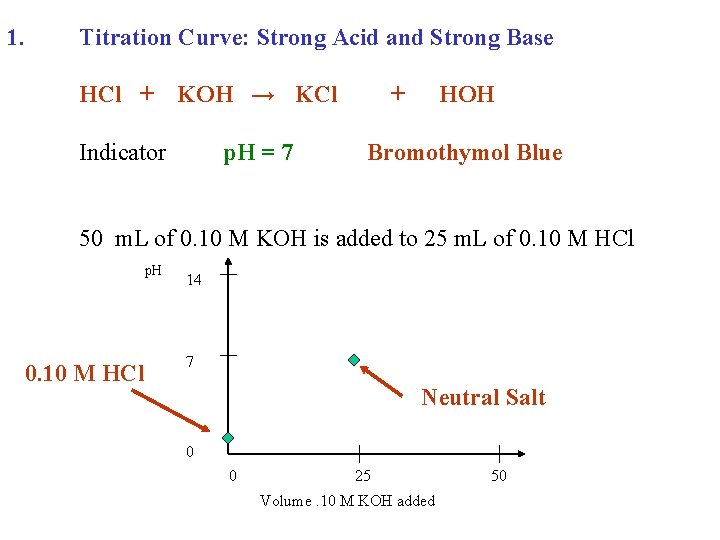
1. Titration Curve: Strong Acid and Strong Base HCl + KOH →

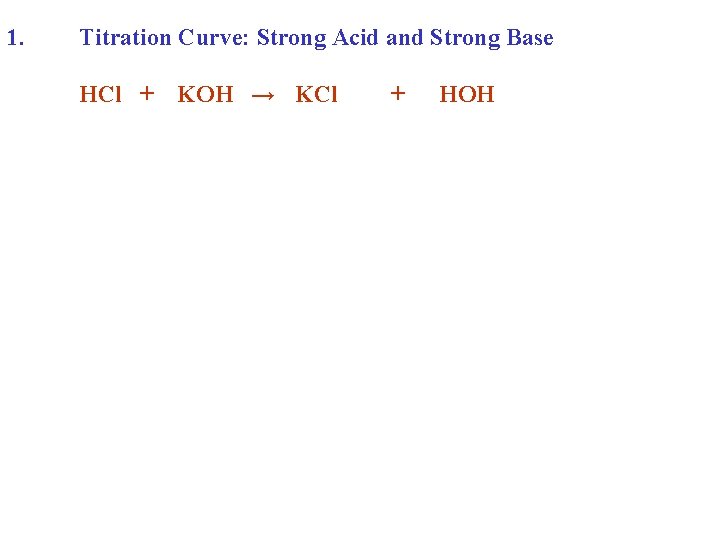
1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl + HOH

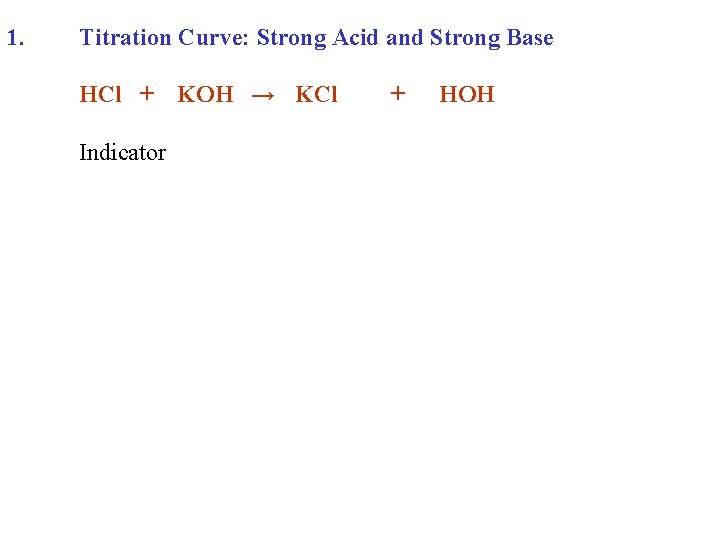
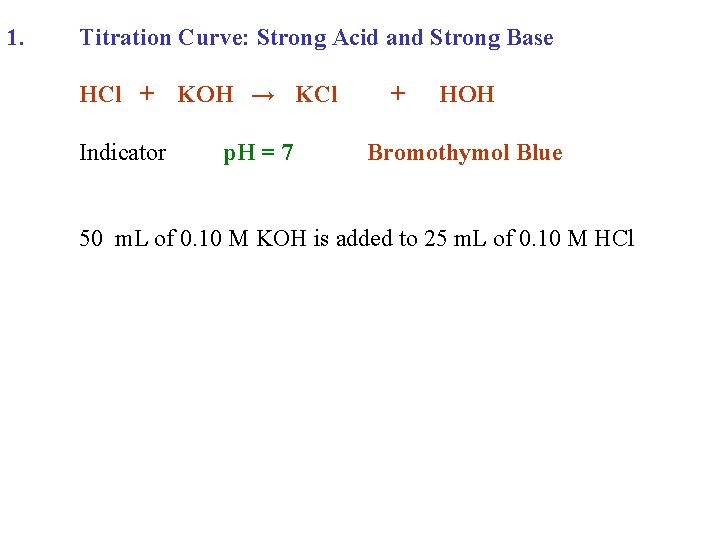
1. Titration Curve: Strong Acid and Strong Base HCl + Indicator KOH → KCl + HOH

1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl Indicator p. H = 7 + HOH Bromothymol Blue

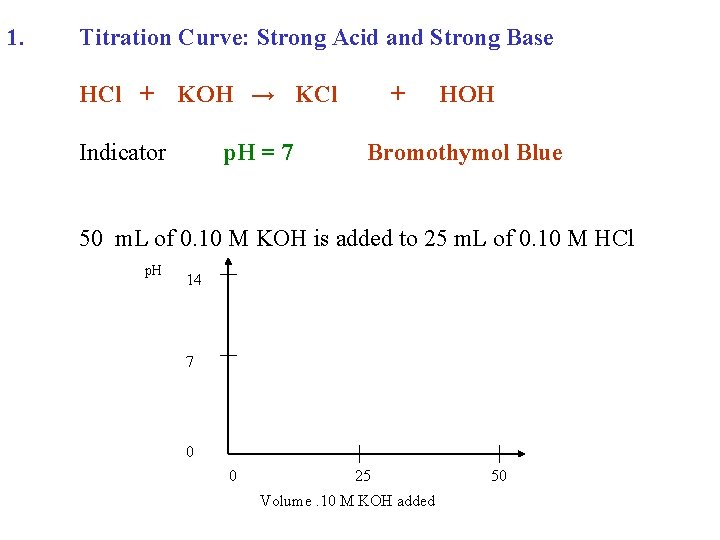
1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl Indicator p. H = 7 + HOH Bromothymol Blue 50 m. L of 0. 10 M KOH is added to 25 m. L of 0. 10 M HCl

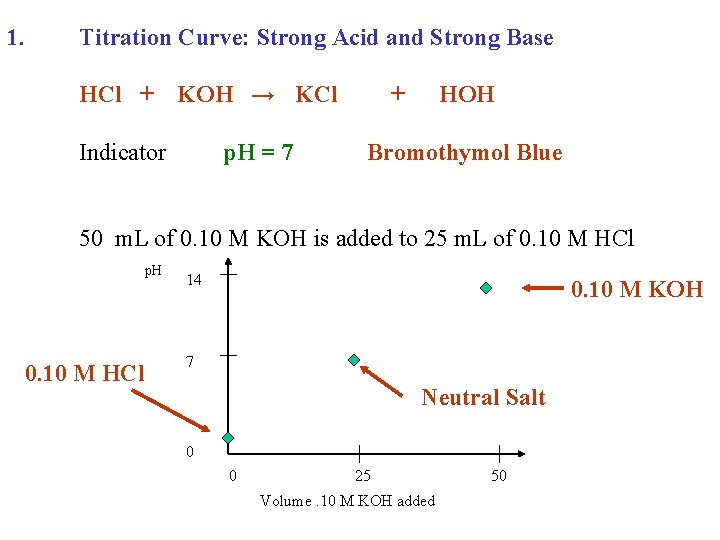
1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl Indicator p. H = 7 + HOH Bromothymol Blue 50 m. L of 0. 10 M KOH is added to 25 m. L of 0. 10 M HCl p. H 14 7 0 0 25 Volume. 10 M KOH added 50

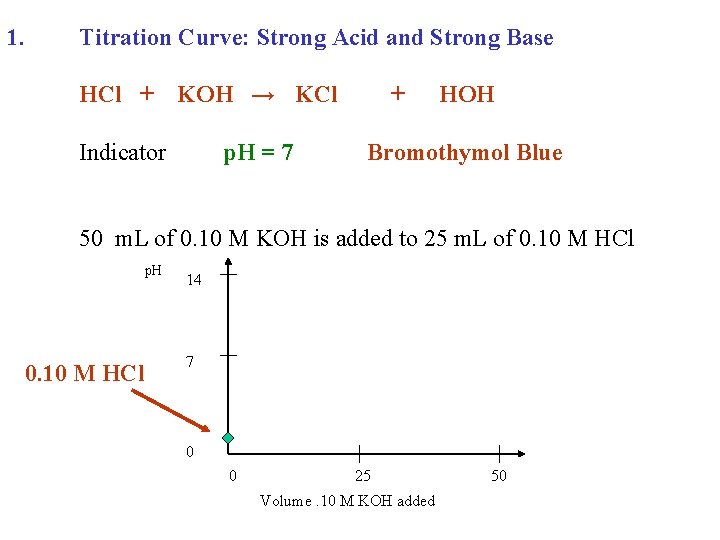
1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl Indicator p. H = 7 + HOH Bromothymol Blue 50 m. L of 0. 10 M KOH is added to 25 m. L of 0. 10 M HCl p. H 0. 10 M HCl 14 7 0 0 25 Volume. 10 M KOH added 50

1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl Indicator p. H = 7 + HOH Bromothymol Blue 50 m. L of 0. 10 M KOH is added to 25 m. L of 0. 10 M HCl p. H 0. 10 M HCl 14 7 Neutral Salt 0 0 25 Volume. 10 M KOH added 50

1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl Indicator p. H = 7 + HOH Bromothymol Blue 50 m. L of 0. 10 M KOH is added to 25 m. L of 0. 10 M HCl p. H 0. 10 M HCl 14 0. 10 M KOH 7 Neutral Salt 0 0 25 Volume. 10 M KOH added 50

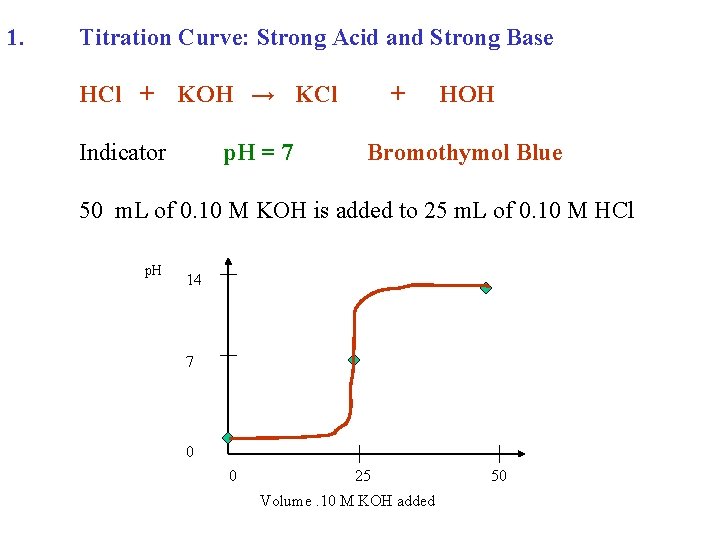
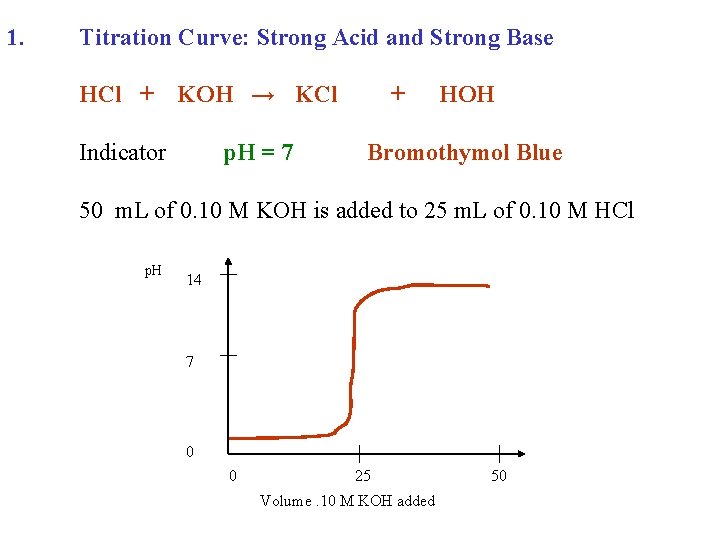
1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl Indicator p. H = 7 + HOH Bromothymol Blue 50 m. L of 0. 10 M KOH is added to 25 m. L of 0. 10 M HCl p. H 14 7 0 0 25 Volume. 10 M KOH added 50

1. Titration Curve: Strong Acid and Strong Base HCl + KOH → KCl Indicator p. H = 7 + HOH Bromothymol Blue 50 m. L of 0. 10 M KOH is added to 25 m. L of 0. 10 M HCl p. H 14 7 0 0 25 Volume. 10 M KOH added 50

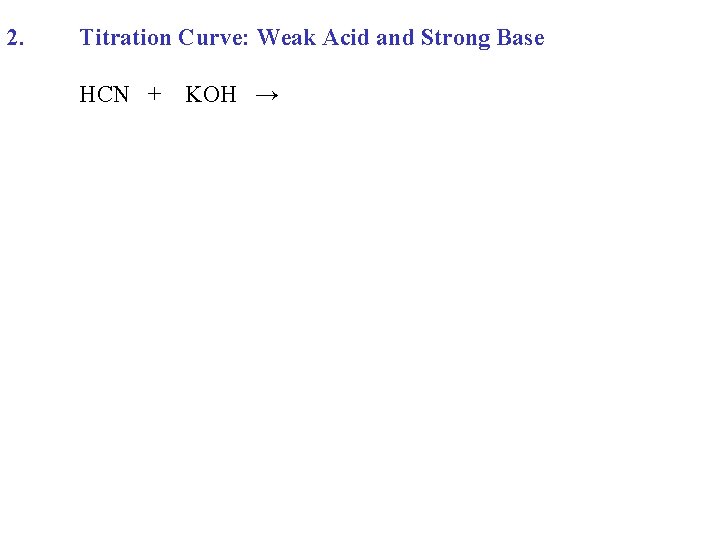
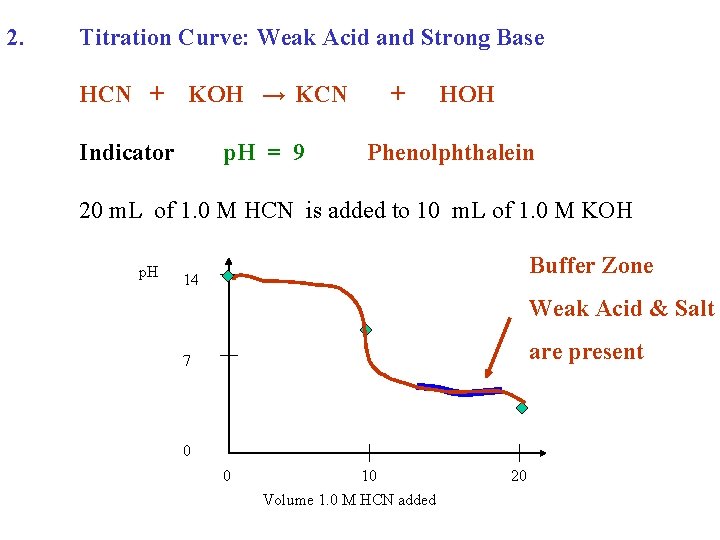
2. Titration Curve: Weak Acid and Strong Base HCN + KOH →

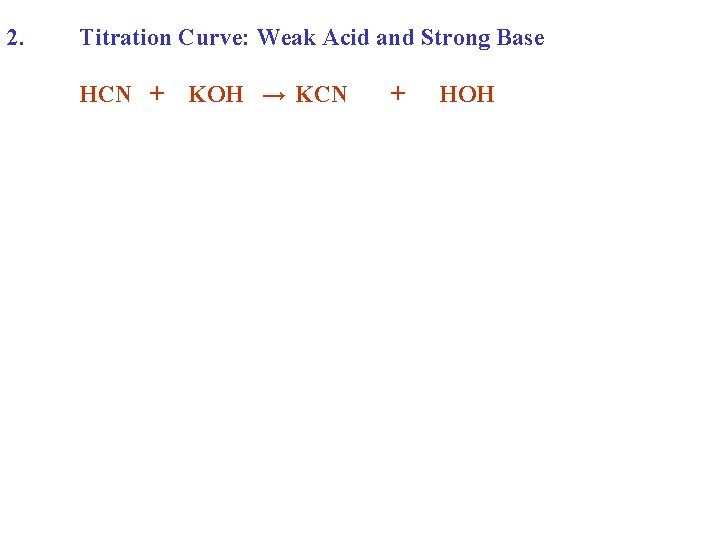
2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN + HOH

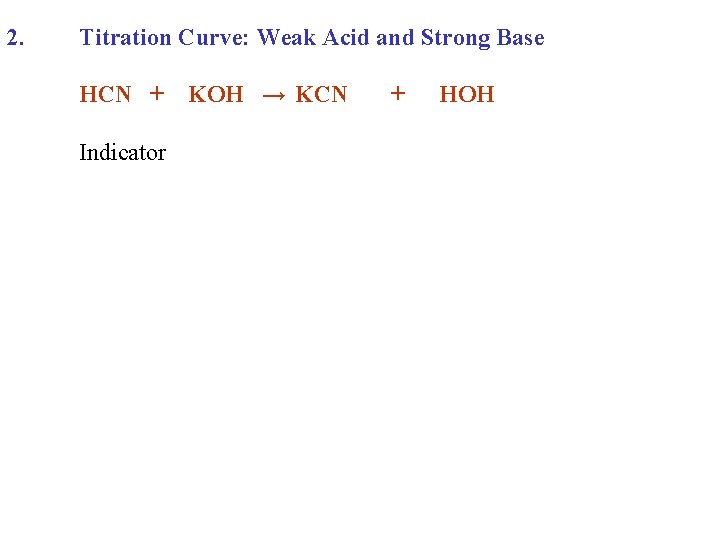
2. Titration Curve: Weak Acid and Strong Base HCN + Indicator KOH → KCN + HOH

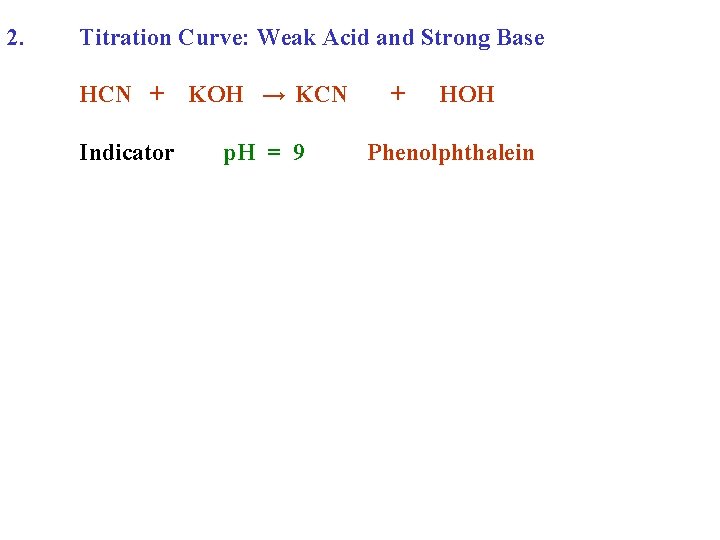
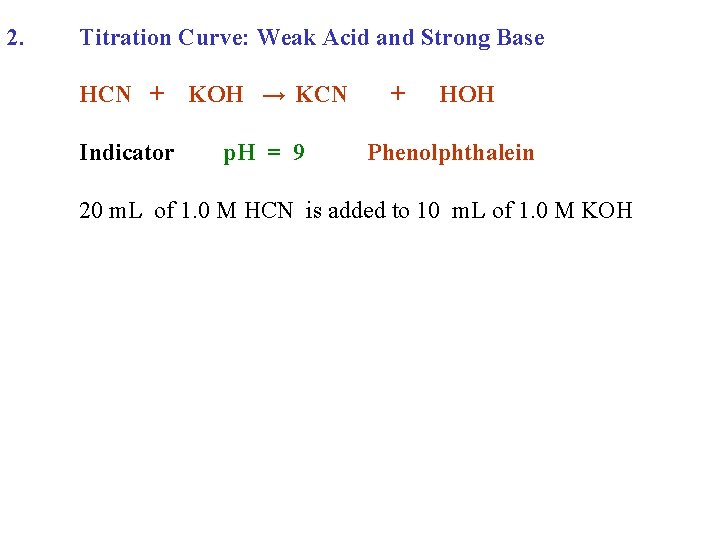
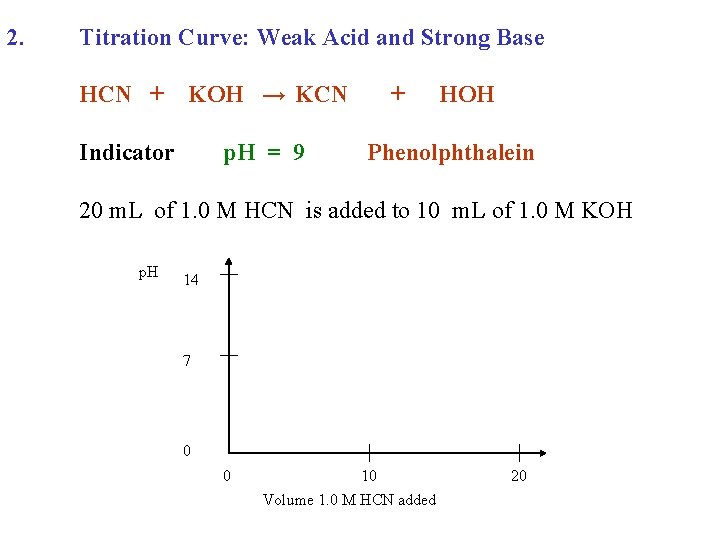
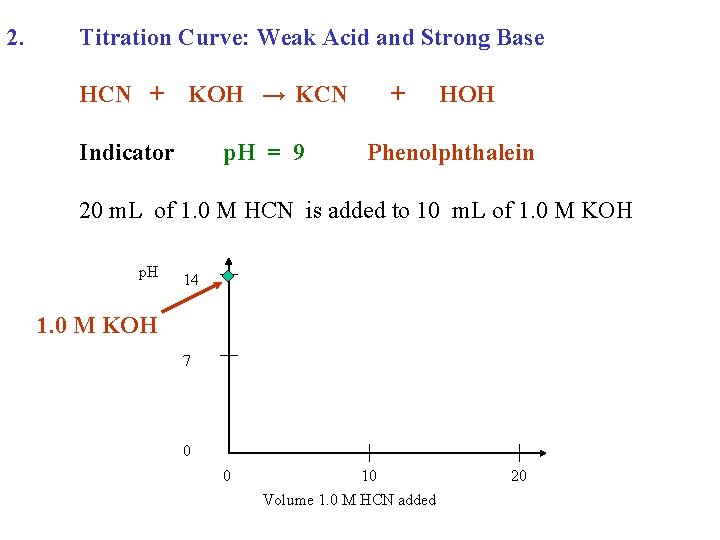
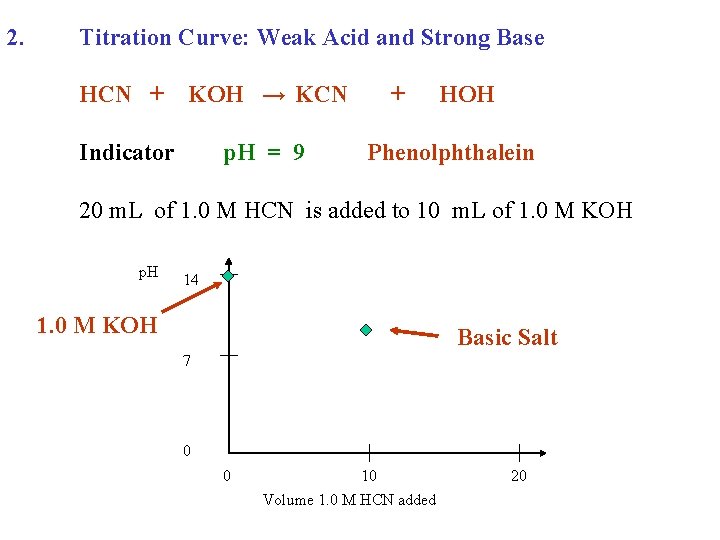
2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein

2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH

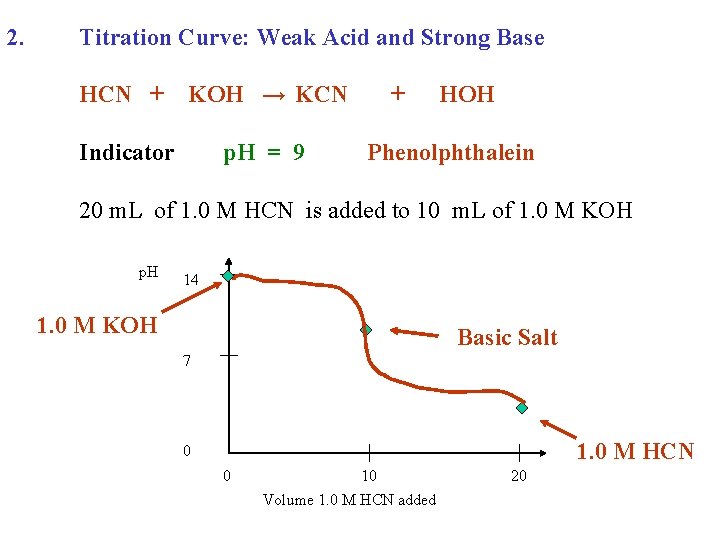
2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH p. H 14 7 0 0 10 Volume 1. 0 M HCN added 20

2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH p. H 14 1. 0 M KOH 7 0 0 10 Volume 1. 0 M HCN added 20

2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH p. H 14 1. 0 M KOH Basic Salt 7 0 0 10 Volume 1. 0 M HCN added 20

2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH p. H 14 1. 0 M KOH Basic Salt 7 1. 0 M HCN 0 0 10 Volume 1. 0 M HCN added 20

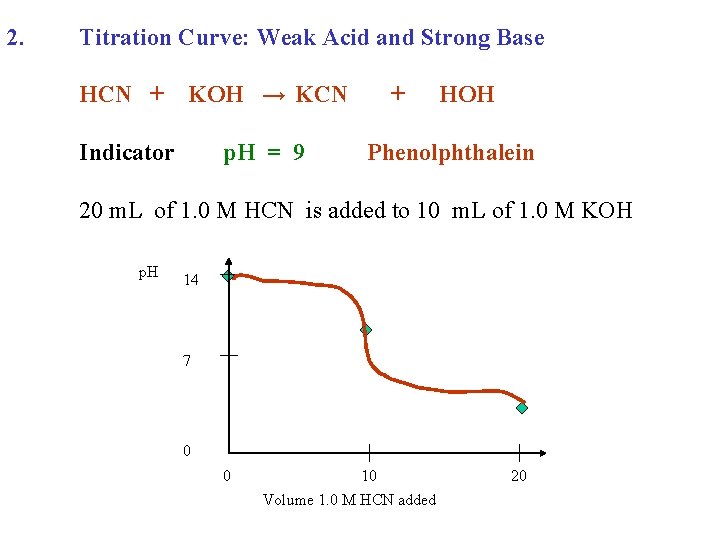
2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH p. H 14 1. 0 M KOH Basic Salt 7 1. 0 M HCN 0 0 10 Volume 1. 0 M HCN added 20

2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH p. H 14 7 0 0 10 Volume 1. 0 M HCN added 20

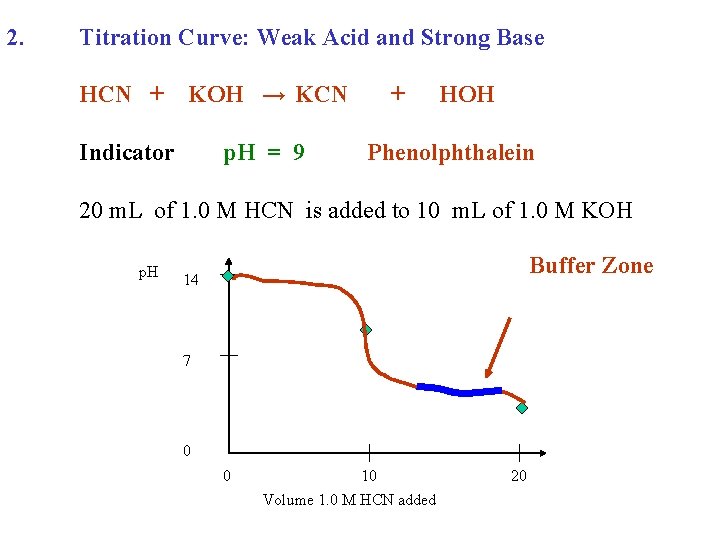
2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH p. H Buffer Zone 14 7 0 0 10 Volume 1. 0 M HCN added 20

2. Titration Curve: Weak Acid and Strong Base HCN + KOH → KCN Indicator p. H = 9 + HOH Phenolphthalein 20 m. L of 1. 0 M HCN is added to 10 m. L of 1. 0 M KOH p. H Buffer Zone 14 Weak Acid & Salt are present 7 0 0 10 Volume 1. 0 M HCN added 20

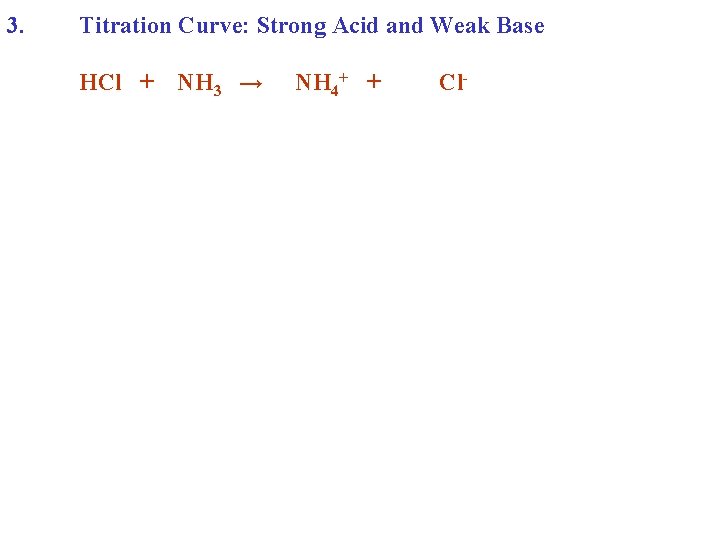
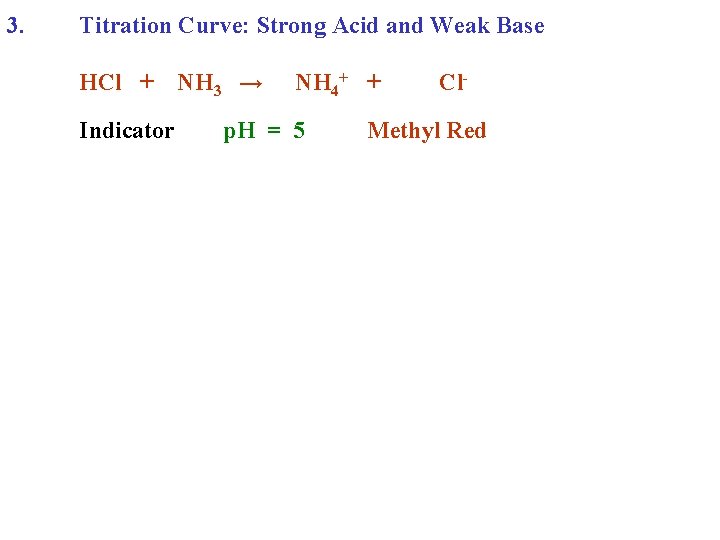
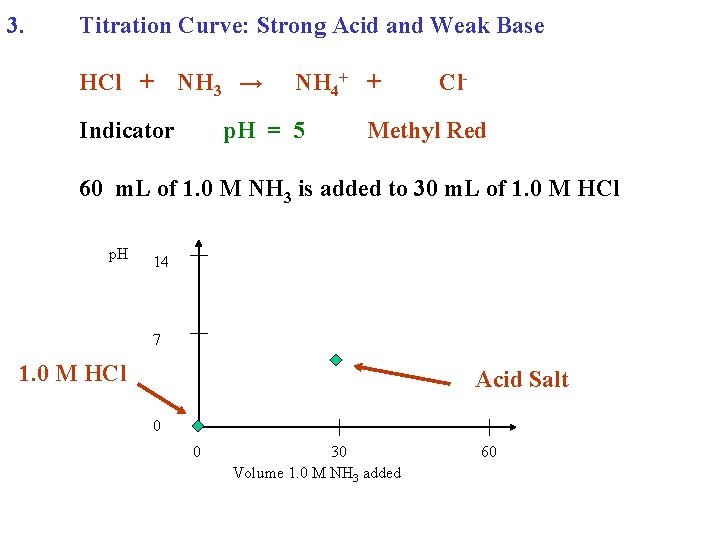
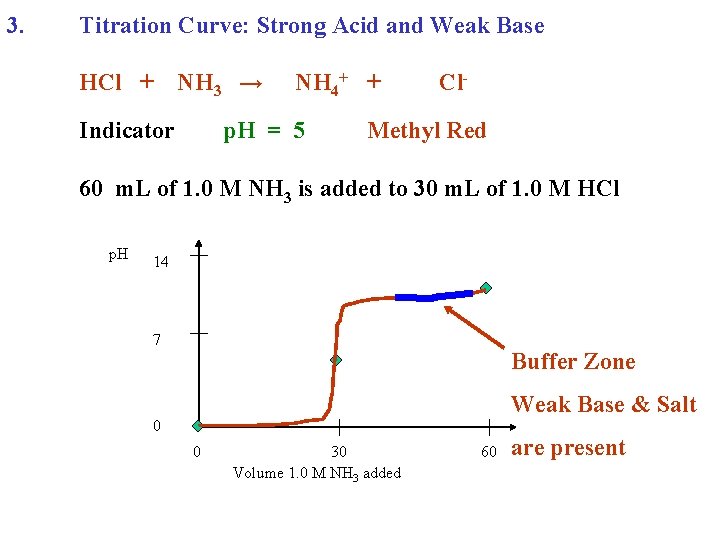
3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 →

3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 → NH 4+ + Cl-

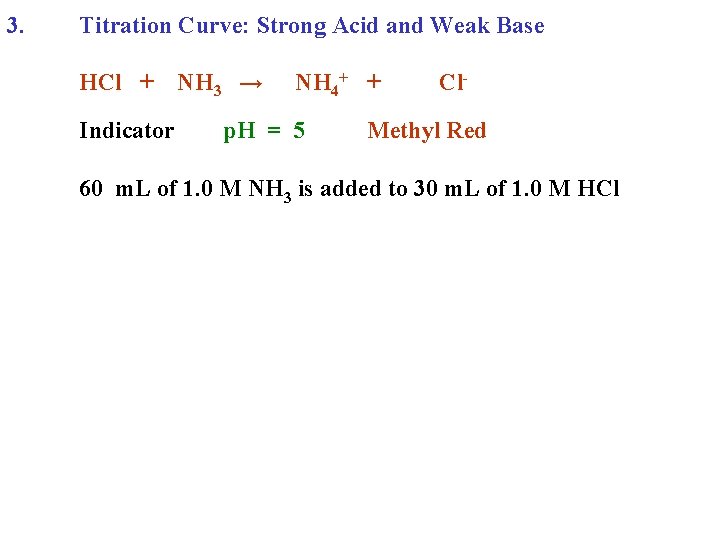
3. Titration Curve: Strong Acid and Weak Base HCl + Indicator NH 3 → NH 4+ + Cl-

3. Titration Curve: Strong Acid and Weak Base HCl + Indicator NH 3 → NH 4+ + p. H = 5 Cl- Methyl Red

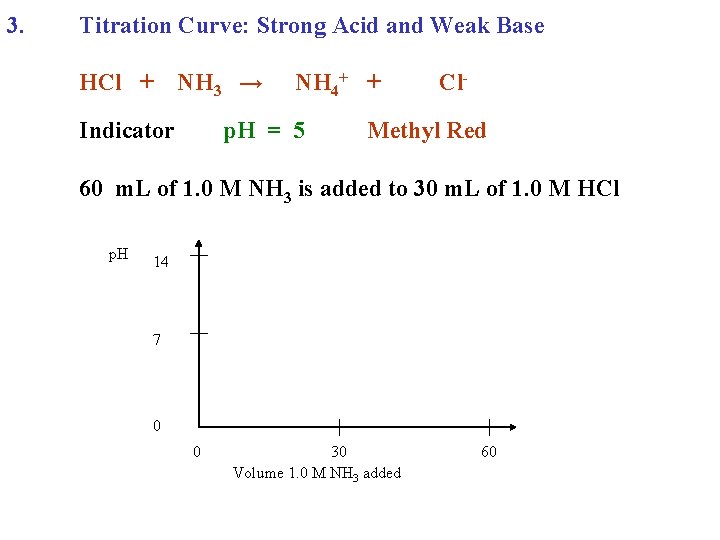
3. Titration Curve: Strong Acid and Weak Base HCl + Indicator NH 3 → NH 4+ + p. H = 5 Cl- Methyl Red 60 m. L of 1. 0 M NH 3 is added to 30 m. L of 1. 0 M HCl

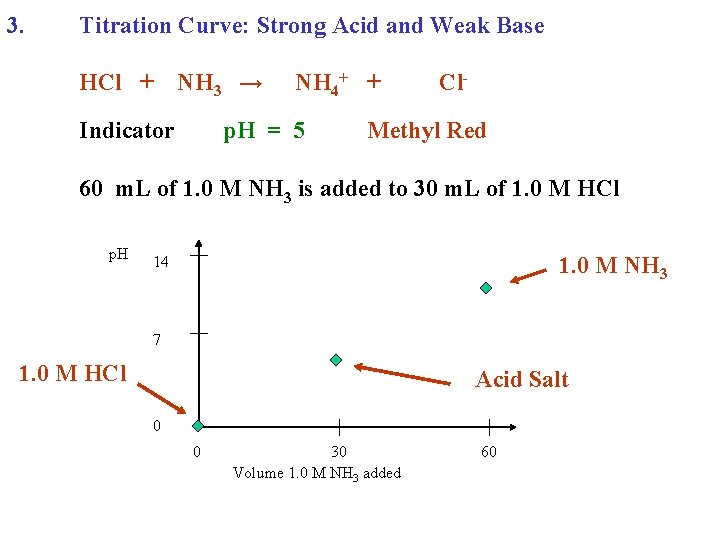
3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 → Indicator NH 4+ + p. H = 5 Cl- Methyl Red 60 m. L of 1. 0 M NH 3 is added to 30 m. L of 1. 0 M HCl p. H 14 7 0 0 30 Volume 1. 0 M NH 3 added 60

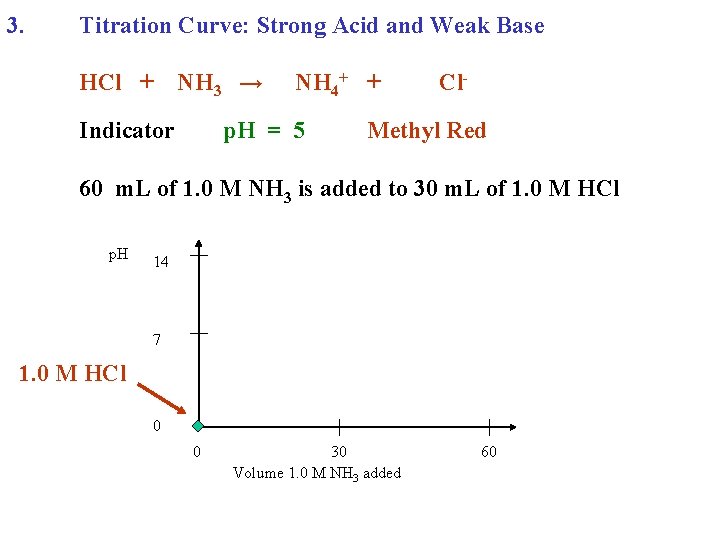
3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 → Indicator NH 4+ + p. H = 5 Cl- Methyl Red 60 m. L of 1. 0 M NH 3 is added to 30 m. L of 1. 0 M HCl p. H 14 7 1. 0 M HCl 0 0 30 Volume 1. 0 M NH 3 added 60

3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 → Indicator NH 4+ + p. H = 5 Cl- Methyl Red 60 m. L of 1. 0 M NH 3 is added to 30 m. L of 1. 0 M HCl p. H 14 7 1. 0 M HCl Acid Salt 0 0 30 Volume 1. 0 M NH 3 added 60

3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 → Indicator NH 4+ + p. H = 5 Cl- Methyl Red 60 m. L of 1. 0 M NH 3 is added to 30 m. L of 1. 0 M HCl p. H 1. 0 M NH 3 14 7 1. 0 M HCl Acid Salt 0 0 30 Volume 1. 0 M NH 3 added 60

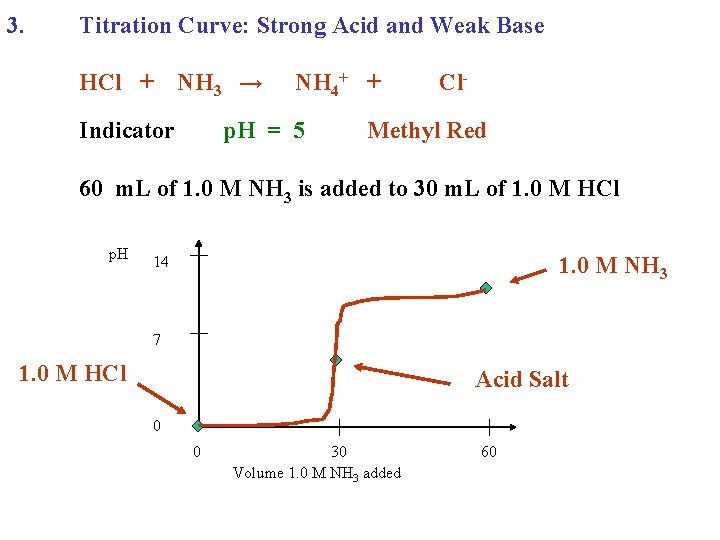
3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 → Indicator NH 4+ + p. H = 5 Cl- Methyl Red 60 m. L of 1. 0 M NH 3 is added to 30 m. L of 1. 0 M HCl p. H 1. 0 M NH 3 14 7 1. 0 M HCl Acid Salt 0 0 30 Volume 1. 0 M NH 3 added 60

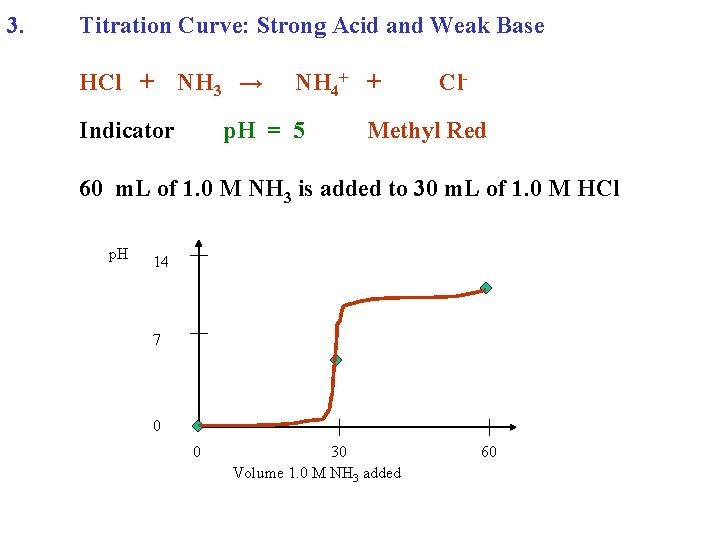
3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 → Indicator NH 4+ + p. H = 5 Cl- Methyl Red 60 m. L of 1. 0 M NH 3 is added to 30 m. L of 1. 0 M HCl p. H 14 7 0 0 30 Volume 1. 0 M NH 3 added 60

3. Titration Curve: Strong Acid and Weak Base HCl + NH 3 → Indicator NH 4+ + p. H = 5 Cl- Methyl Red 60 m. L of 1. 0 M NH 3 is added to 30 m. L of 1. 0 M HCl p. H 14 7 Buffer Zone Weak Base & Salt 0 0 30 Volume 1. 0 M NH 3 added 60 are present

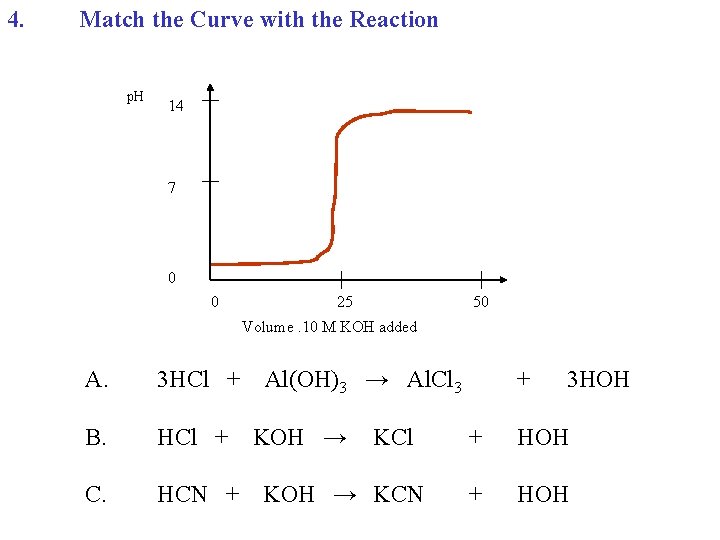
4. Match the Curve with the Reaction p. H 14 7 0 0 25 50 Volume. 10 M KOH added A. 3 HCl + B. HCl + C. HCN + Al(OH)3 → Al. Cl 3 KOH → KCl KOH → KCN + 3 HOH + HOH

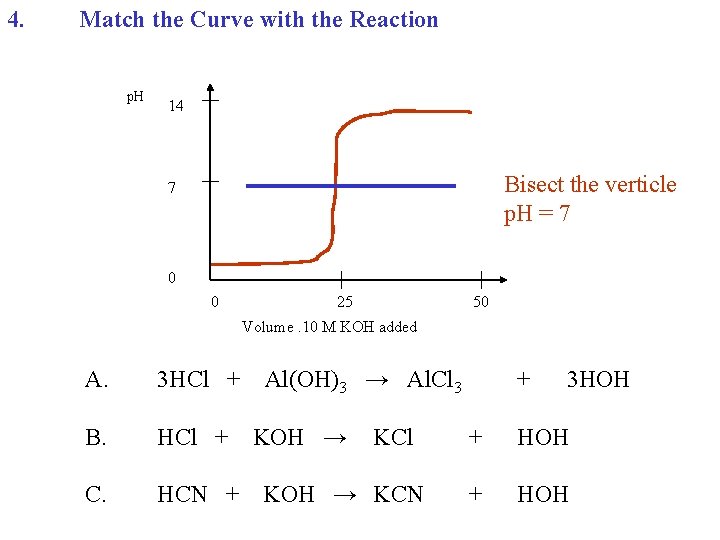
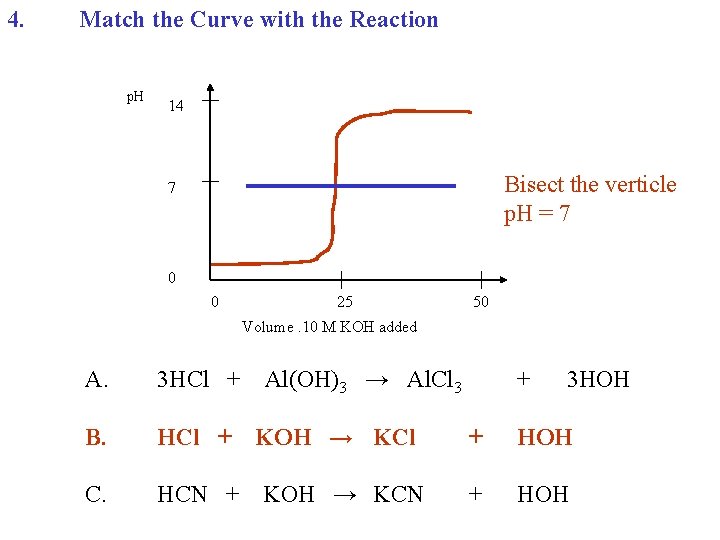
4. Match the Curve with the Reaction p. H 14 Bisect the verticle p. H = 7 7 0 0 25 50 Volume. 10 M KOH added A. 3 HCl + B. HCl + C. HCN + Al(OH)3 → Al. Cl 3 KOH → KCl KOH → KCN + 3 HOH + HOH

4. Match the Curve with the Reaction p. H 14 Bisect the verticle p. H = 7 7 0 0 25 50 Volume. 10 M KOH added A. 3 HCl + B. HCl + C. HCN + Al(OH)3 → Al. Cl 3 KOH → KCl KOH → KCN + 3 HOH + HOH

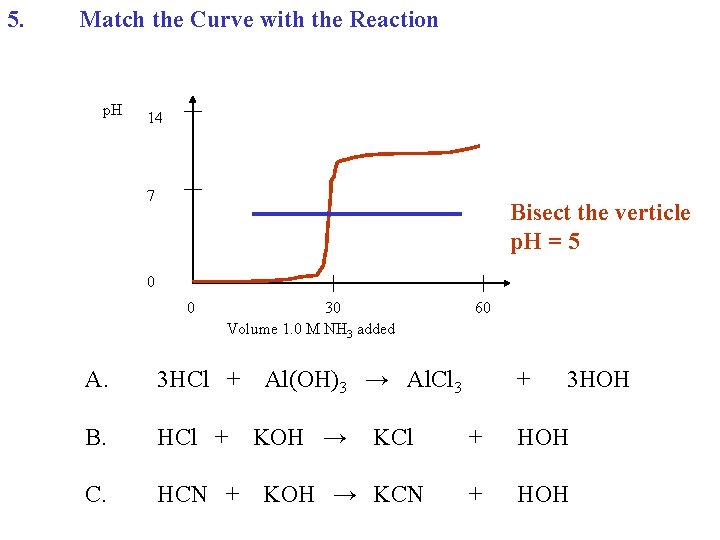
5. Match the Curve with the Reaction p. H 14 7 0 0 30 Volume 1. 0 M NH 3 added A. 3 HCl + B. HCl + C. HCN + 60 Al(OH)3 → Al. Cl 3 KOH → KCl KOH → KCN + 3 HOH + HOH

5. Match the Curve with the Reaction p. H 14 7 Bisect the verticle p. H = 5 0 0 30 Volume 1. 0 M NH 3 added A. 3 HCl + B. HCl + C. HCN + 60 Al(OH)3 → Al. Cl 3 KOH → KCl KOH → KCN + 3 HOH + HOH

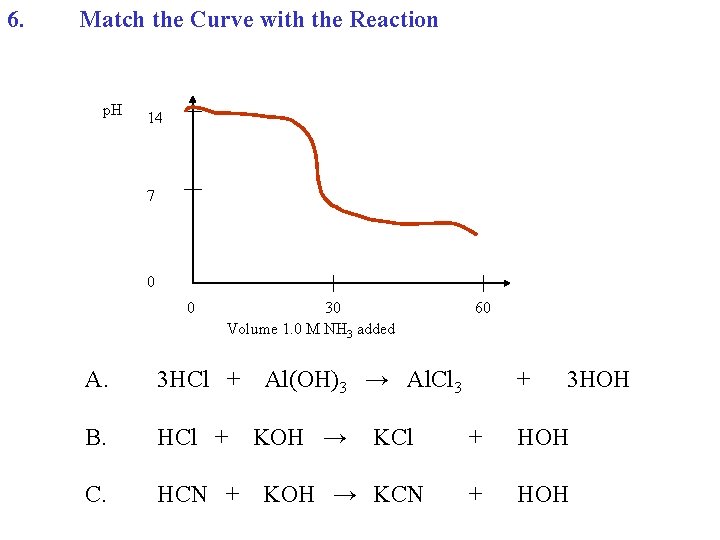
6. Match the Curve with the Reaction p. H 14 7 0 0 30 Volume 1. 0 M NH 3 added A. 3 HCl + B. HCl + C. HCN + 60 Al(OH)3 → Al. Cl 3 KOH → KCl KOH → KCN + 3 HOH + HOH

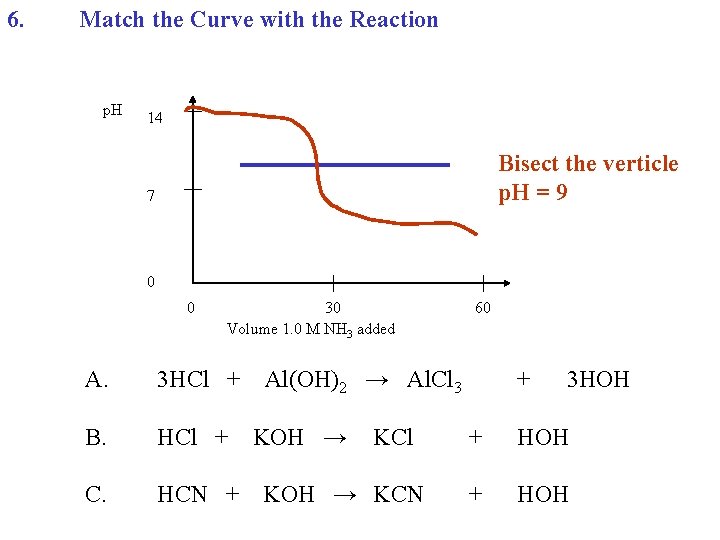
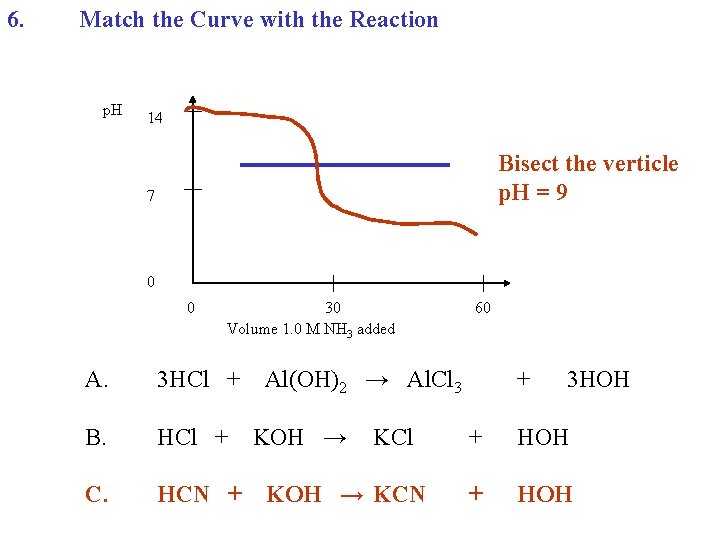
6. Match the Curve with the Reaction p. H 14 Bisect the verticle p. H = 9 7 0 0 30 Volume 1. 0 M NH 3 added A. 3 HCl + B. HCl + C. HCN + 60 Al(OH)2 → Al. Cl 3 KOH → KCl KOH → KCN + 3 HOH + HOH

6. Match the Curve with the Reaction p. H 14 Bisect the verticle p. H = 9 7 0 0 30 Volume 1. 0 M NH 3 added A. 3 HCl + B. HCl + C. HCN + 60 Al(OH)2 → Al. Cl 3 KOH → KCl KOH → KCN + 3 HOH + HOH