Theory of NMR Spectroscopy Dr Ekta Khosla Department


- Slides: 39

Theory of NMR Spectroscopy Dr Ekta Khosla Department of Chemistry

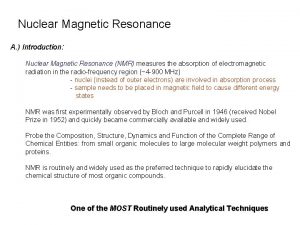
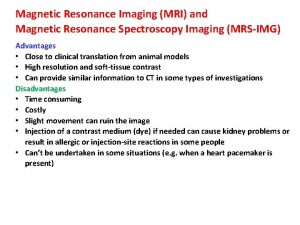
Nuclear Magnetic Resonance (NMR) Introduction: Nuclear Magnetic Resonance (NMR) measures the absorption of electromagnetic radiation in the radio-frequency region (~4 -900 MHz) - nuclei (instead of outer electrons) are involved in absorption process - sample needs to be placed in magnetic field to cause different energy states NMR was first experimentally observed by Bloch and Purcell in 1946 (received Nobel Prize in 1952) and quickly became commercially available and widely used. Probe the Composition, Structure, Dynamics and Function of the Complete Range of Chemical Entities: from small organic molecules to large molecular weight polymers and proteins. NMR is routinely and widely used as the preferred technique to rapidly elucidate the chemical structure of most organic compounds.

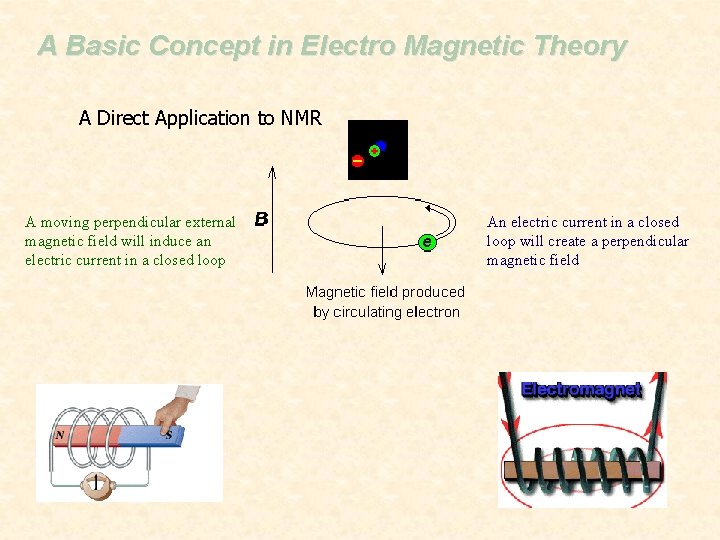
A Basic Concept in Electro Magnetic Theory A Direct Application to NMR A moving perpendicular external magnetic field will induce an electric current in a closed loop An electric current in a closed loop will create a perpendicular magnetic field

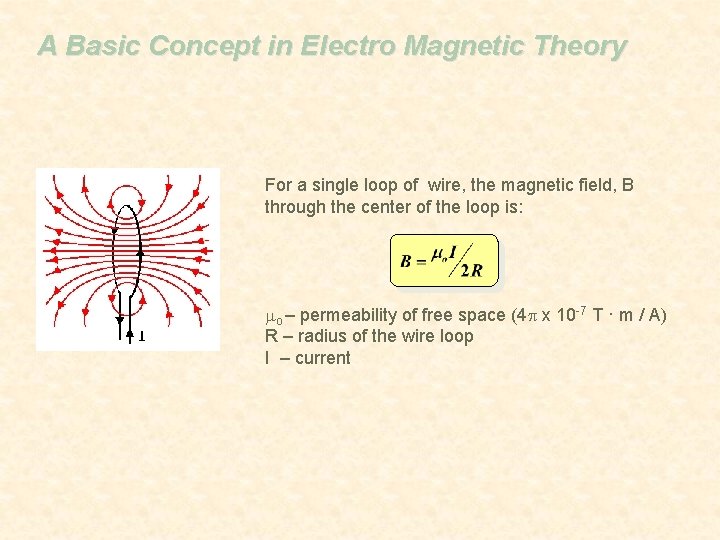
A Basic Concept in Electro Magnetic Theory For a single loop of wire, the magnetic field, B through the center of the loop is: mo – permeability of free space (4 p x 10 -7 T · m / A) R – radius of the wire loop I – current

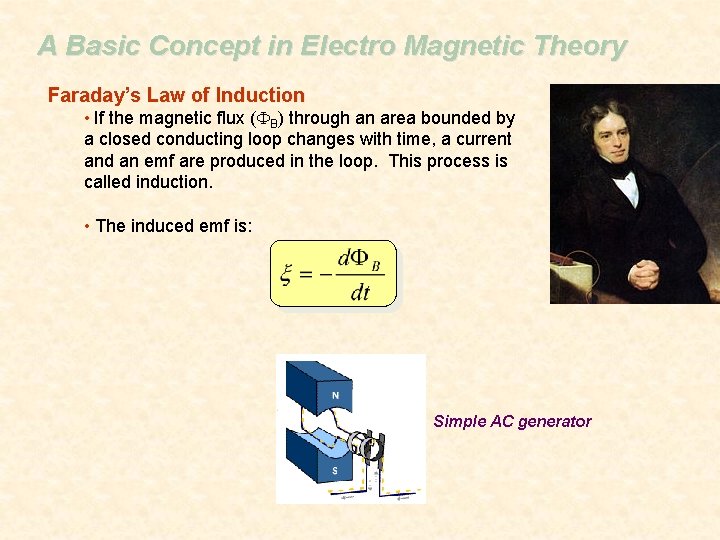
A Basic Concept in Electro Magnetic Theory Faraday’s Law of Induction • If the magnetic flux (FB) through an area bounded by a closed conducting loop changes with time, a current and an emf are produced in the loop. This process is called induction. • The induced emf is: Simple AC generator

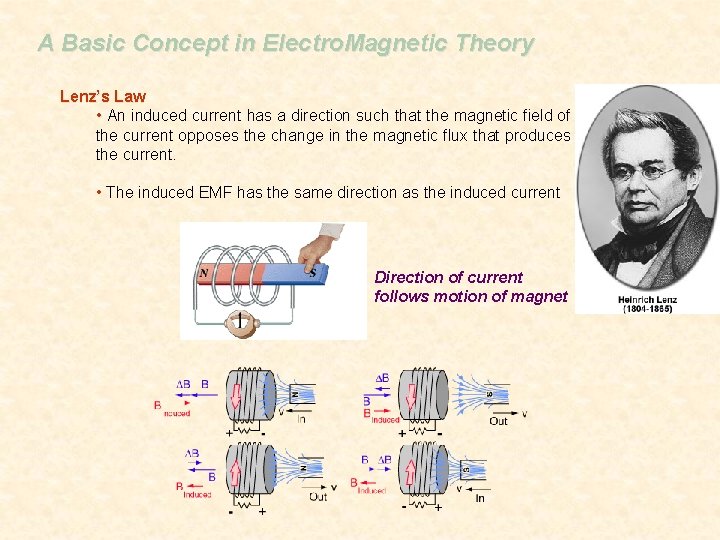
A Basic Concept in Electro. Magnetic Theory Lenz’s Law • An induced current has a direction such that the magnetic field of the current opposes the change in the magnetic flux that produces the current. • The induced EMF has the same direction as the induced current Direction of current follows motion of magnet

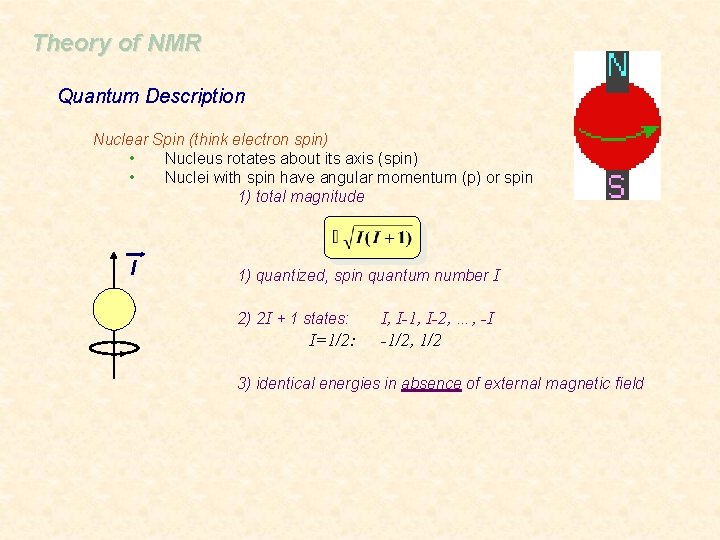
Theory of NMR Quantum Description Nuclear Spin (think electron spin) • Nucleus rotates about its axis (spin) • Nuclei with spin have angular momentum (p) or spin 1) total magnitude l 1) quantized, spin quantum number I 2) 2 I + 1 states: I=1/2: I, I-1, I-2, …, -I -1/2, 1/2 3) identical energies in absence of external magnetic field

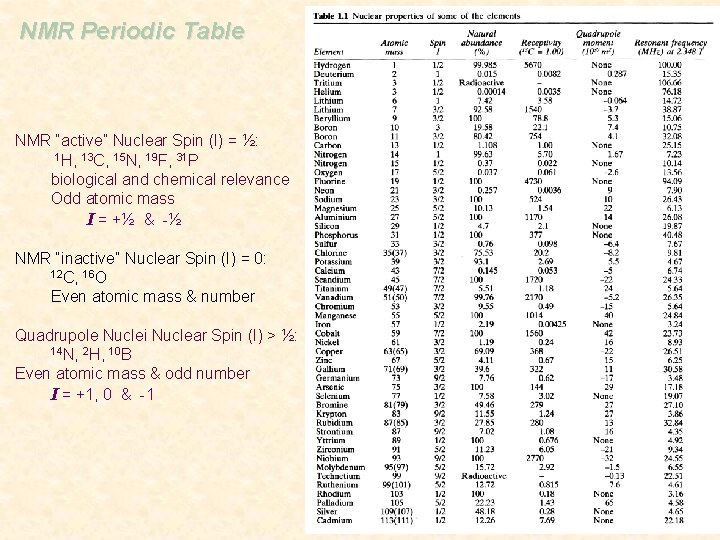
NMR Periodic Table NMR “active” Nuclear Spin (I) = ½: 1 H, 13 C, 15 N, 19 F, 31 P biological and chemical relevance Odd atomic mass I = +½ & -½ NMR “inactive” Nuclear Spin (I) = 0: 12 C, 16 O Even atomic mass & number Quadrupole Nuclei Nuclear Spin (I) > ½: 14 N, 2 H, 10 B Even atomic mass & odd number I = +1, 0 & -1

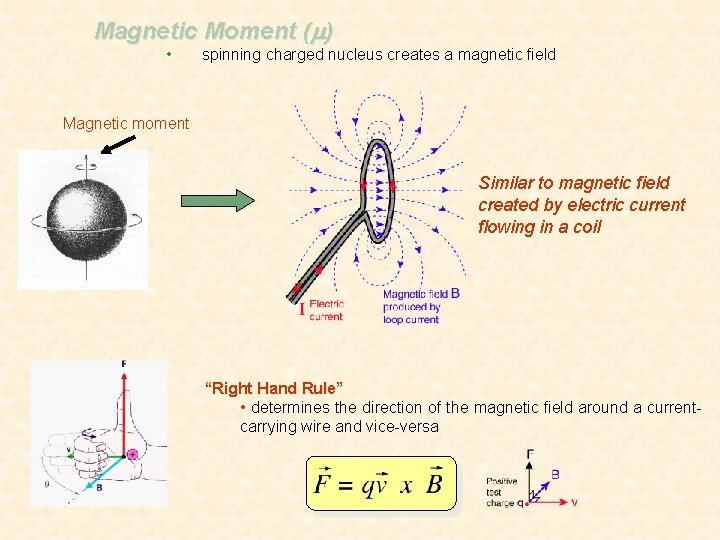
Magnetic Moment (m) • spinning charged nucleus creates a magnetic field Magnetic moment Similar to magnetic field created by electric current flowing in a coil “Right Hand Rule” • determines the direction of the magnetic field around a currentcarrying wire and vice-versa

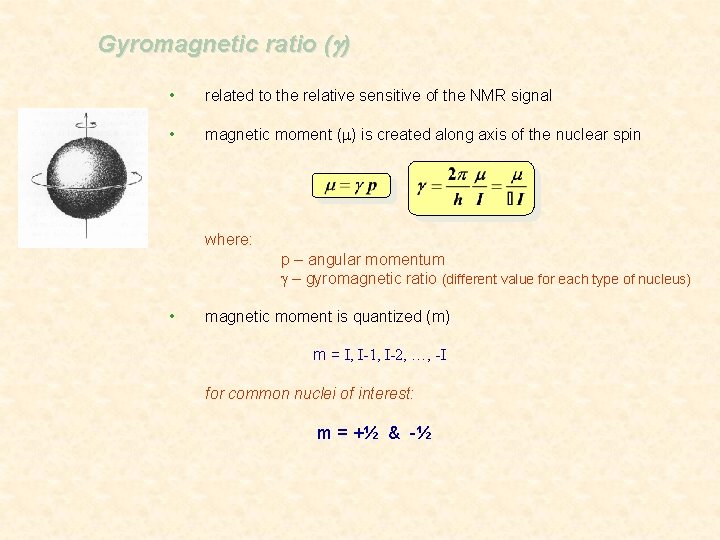
Gyromagnetic ratio (g) • related to the relative sensitive of the NMR signal • magnetic moment (m) is created along axis of the nuclear spin where: p – angular momentum g – gyromagnetic ratio (different value for each type of nucleus) • magnetic moment is quantized (m) m = I, I-1, I-2, …, -I for common nuclei of interest: m = +½ & -½

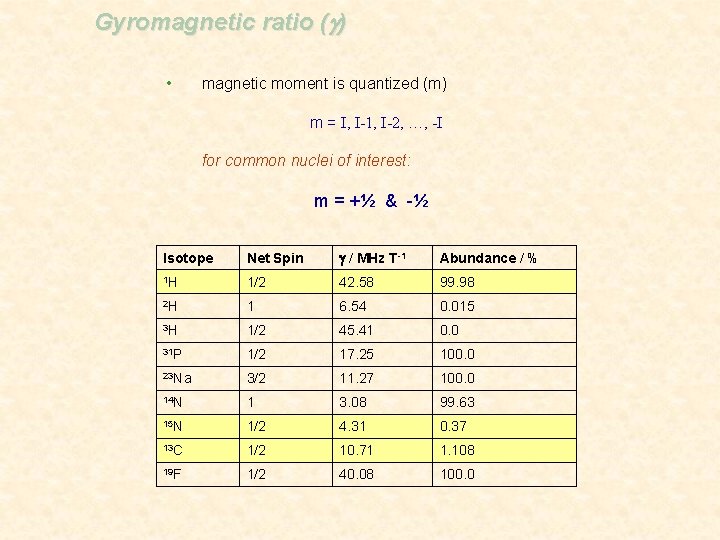
Gyromagnetic ratio (g) • magnetic moment is quantized (m) m = I, I-1, I-2, …, -I for common nuclei of interest: m = +½ & -½ Isotope Net Spin g / MHz T-1 Abundance / % 1 H 1/2 42. 58 99. 98 2 H 1 6. 54 0. 015 3 H 1/2 45. 41 0. 0 31 P 1/2 17. 25 100. 0 23 Na 3/2 11. 27 100. 0 14 N 1 3. 08 99. 63 15 N 1/2 4. 31 0. 37 13 C 1/2 10. 71 1. 108 19 F 1/2 40. 08 100. 0

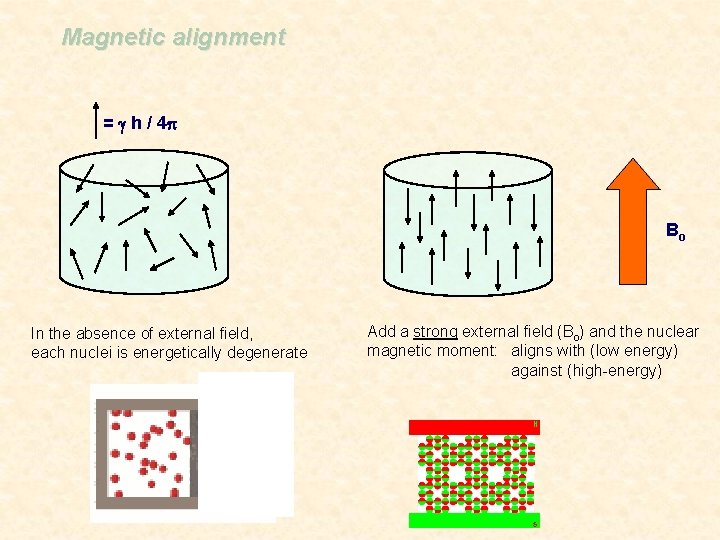
Magnetic alignment = g h / 4 p Bo In the absence of external field, each nuclei is energetically degenerate Add a strong external field (Bo) and the nuclear magnetic moment: aligns with (low energy) against (high-energy)

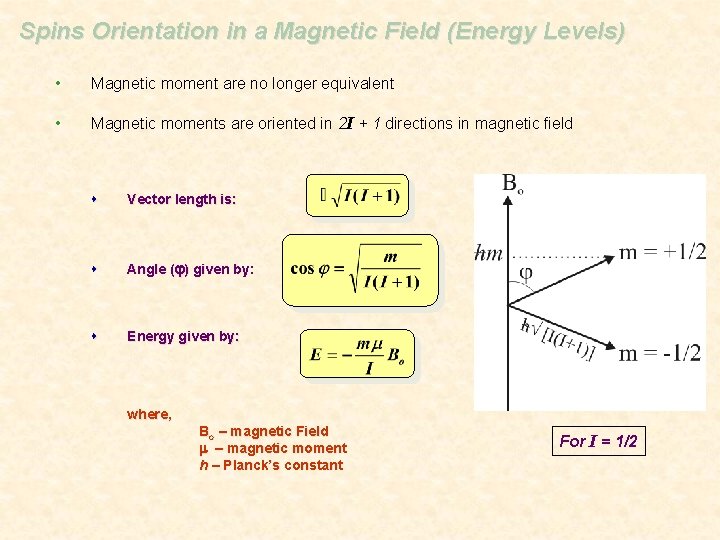
Spins Orientation in a Magnetic Field (Energy Levels) • Magnetic moment are no longer equivalent • Magnetic moments are oriented in 2 I + 1 directions in magnetic field s Vector length is: s Angle (j) given by: s Energy given by: where, Bo – magnetic Field m – magnetic moment h – Planck’s constant For I = 1/2

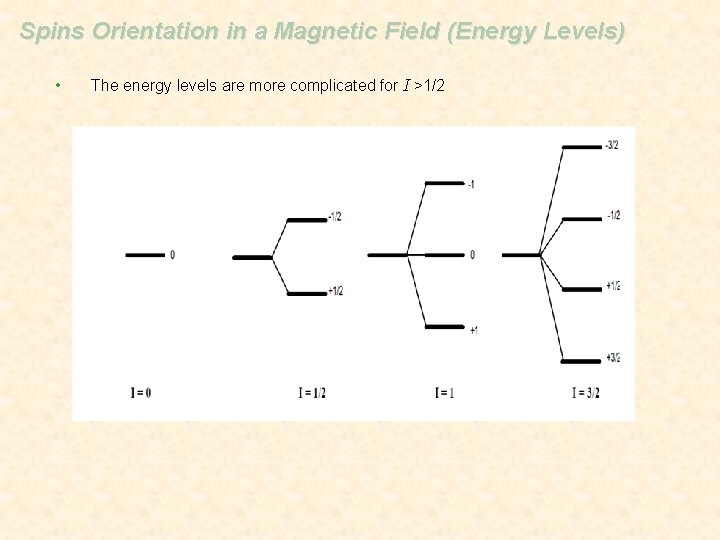
Spins Orientation in a Magnetic Field (Energy Levels) • The energy levels are more complicated for I >1/2

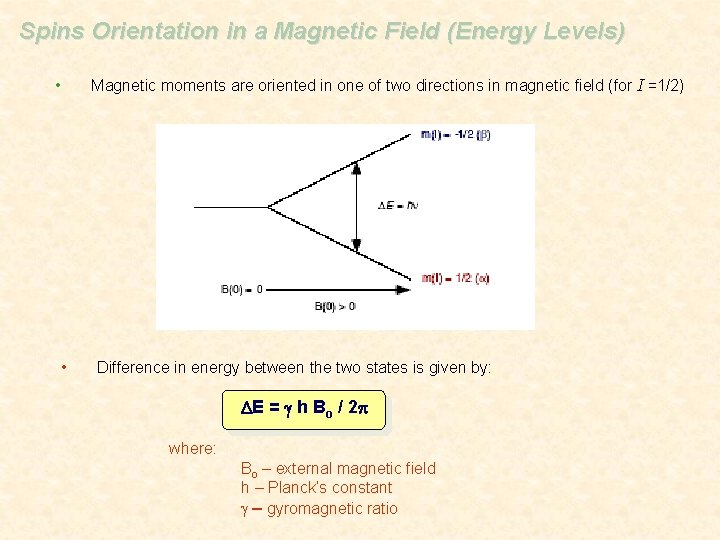
Spins Orientation in a Magnetic Field (Energy Levels) Magnetic moments are oriented in one of two directions in magnetic field (for I =1/2) • • Difference in energy between the two states is given by: DE = g h Bo / 2 p where: Bo – external magnetic field h – Planck’s constant g – gyromagnetic ratio

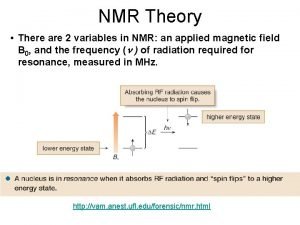
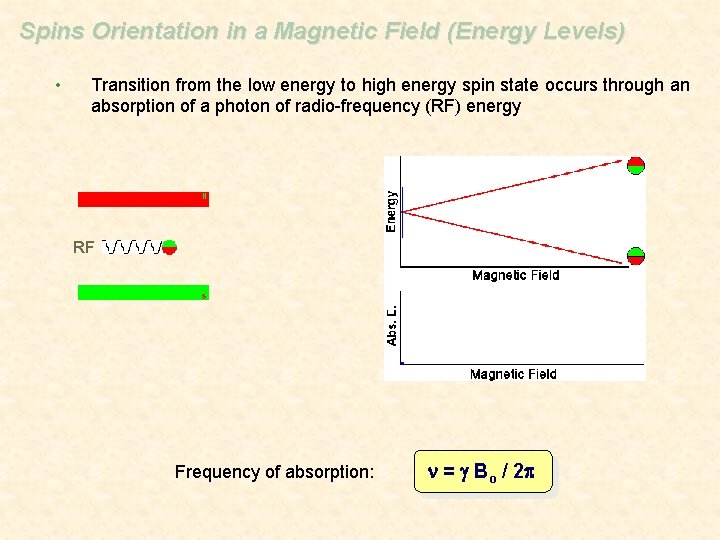
Spins Orientation in a Magnetic Field (Energy Levels) • Transition from the low energy to high energy spin state occurs through an absorption of a photon of radio-frequency (RF) energy RF Frequency of absorption: n = g Bo / 2 p

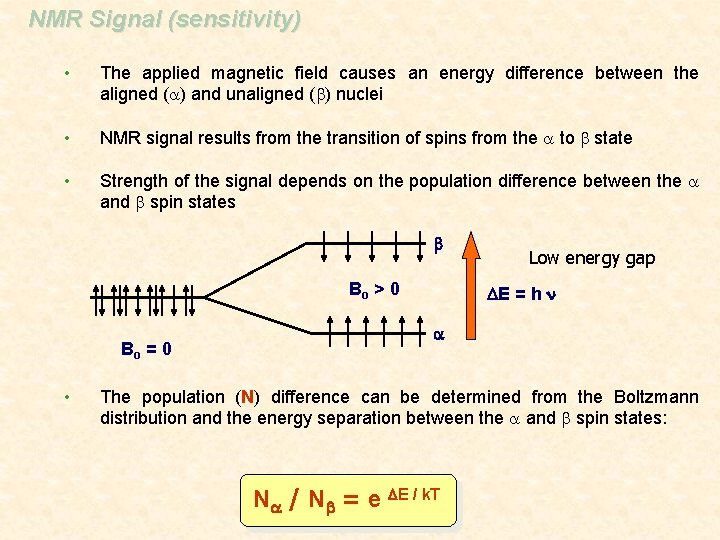
NMR Signal (sensitivity) • The applied magnetic field causes an energy difference between the aligned (a) and unaligned (b) nuclei • NMR signal results from the transition of spins from the a to b state • Strength of the signal depends on the population difference between the a and b spin states b Bo > 0 Bo = 0 • Low energy gap DE = h n a The population (N) difference can be determined from the Boltzmann distribution and the energy separation between the a and b spin states: Na / Nb = e DE / k. T

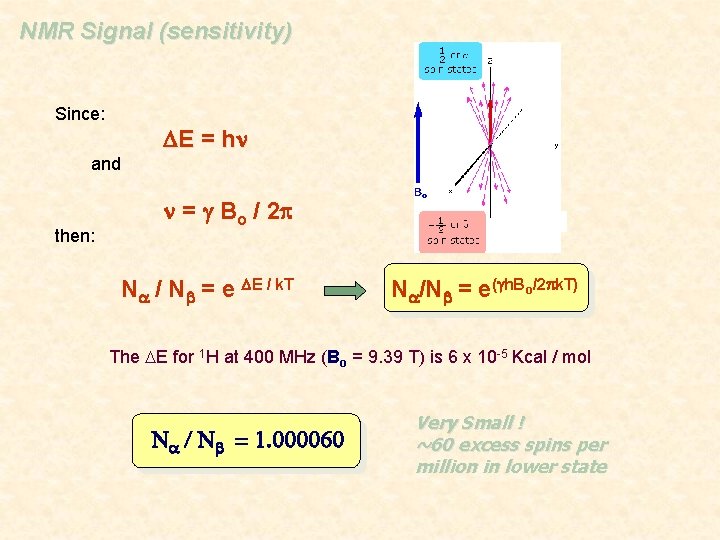
NMR Signal (sensitivity) Since: DE = hn and then: n = g Bo / 2 p Na / Nb = e DE / k. T Na/Nb = e(gh. Bo/2 pk. T) The DE for 1 H at 400 MHz (Bo = 9. 39 T) is 6 x 10 -5 Kcal / mol Na / Nb = 1. 000060 Very Small ! ~60 excess spins per million in lower state

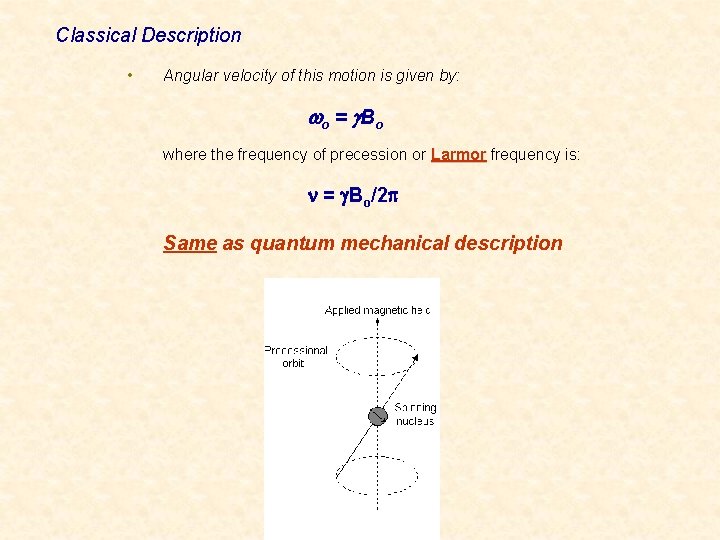
Classical Description • Angular velocity of this motion is given by: wo = g B o where the frequency of precession or Larmor frequency is: Larmor n = g. Bo/2 p Same as quantum mechanical description

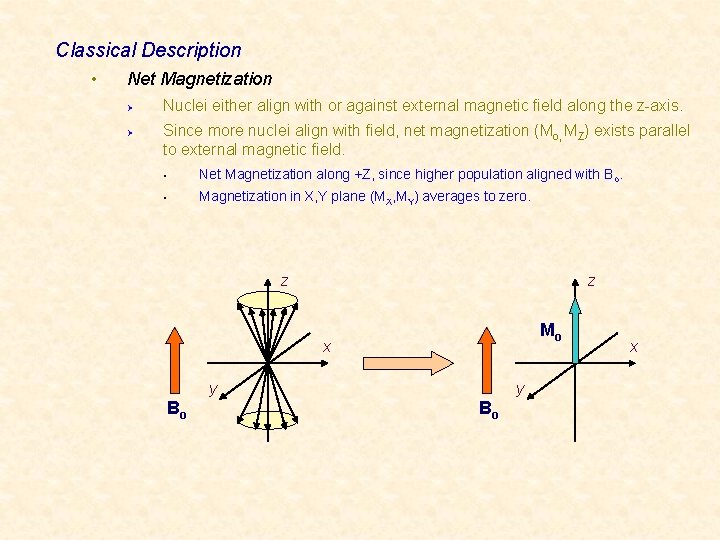
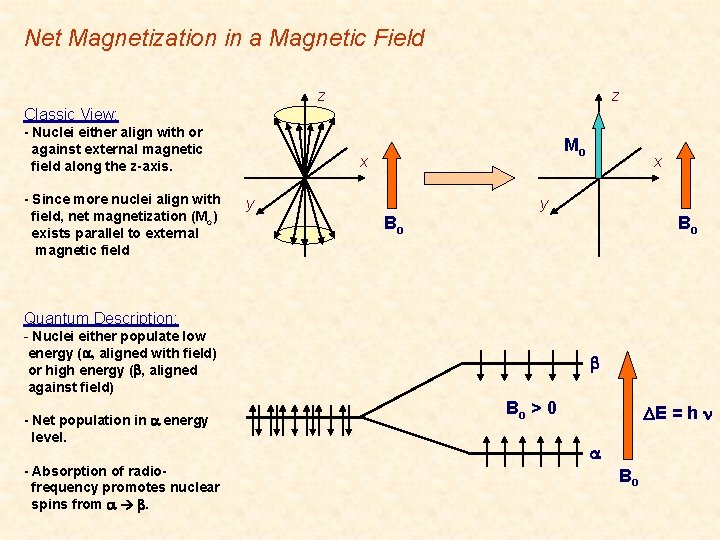
Classical Description • Net Magnetization Ø Ø Nuclei either align with or against external magnetic field along the z-axis. Since more nuclei align with field, net magnetization (Mo, MZ) exists parallel to external magnetic field. § Net Magnetization along +Z, since higher population aligned with Bo. § Magnetization in X, Y plane (MX, MY) averages to zero. z z Mo x y Bo x

Net Magnetization in a Magnetic Field z z Classic View: - Nuclei either align with or against external magnetic field along the z-axis. - Since more nuclei align with field, net magnetization (Mo) exists parallel to external magnetic field Mo x y Bo Bo Quantum Description: - Nuclei either populate low energy (a, aligned with field) or high energy (b, aligned against field) - Net population in a energy level. - Absorption of radio- frequency promotes nuclear spins from a b. b Bo > 0 DE = h n a Bo

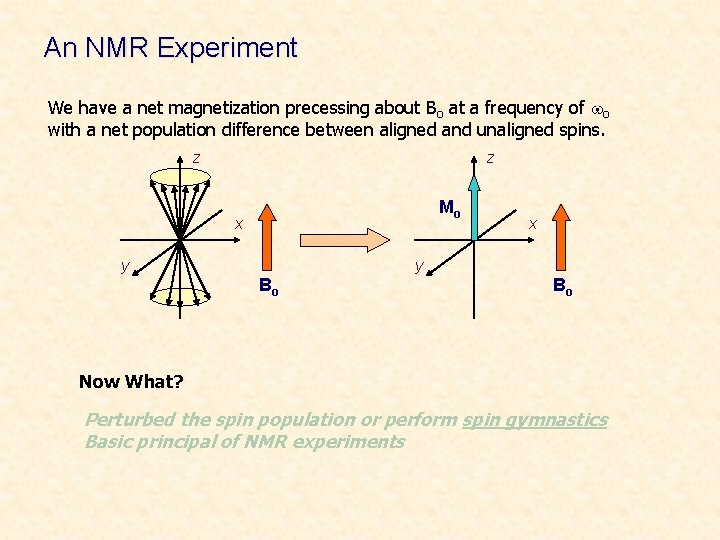
An NMR Experiment We have a net magnetization precessing about Bo at a frequency of wo with a net population difference between aligned and unaligned spins. z z Mo x y Bo Bo Now What? Perturbed the spin population or perform spin gymnastics Basic principal of NMR experiments

The Basic 1 D NMR Experimental details will effect the NMR spectra and the corresponding interpretation

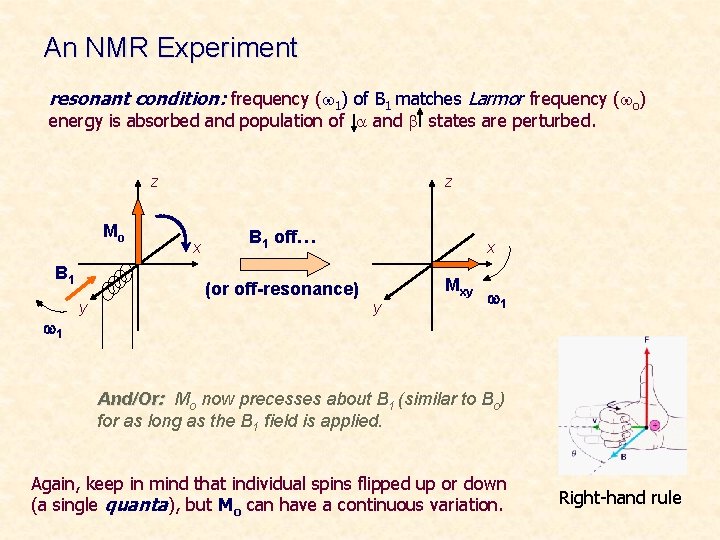
An NMR Experiment resonant condition: frequency (w 1) of B 1 matches Larmor frequency (wo) energy is absorbed and population of a and b states are perturbed. z Mo B 1 w 1 y z x B 1 off… (or off-resonance) x Mxy y w 1 And/Or: Mo now precesses about B 1 (similar to Bo) for as long as the B 1 field is applied. Again, keep in mind that individual spins flipped up or down (a single quanta), but Mo can have a continuous variation. Right-hand rule

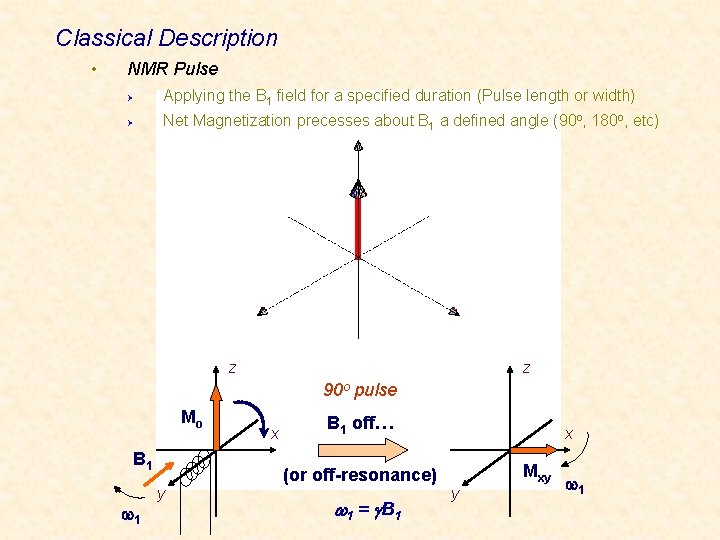
Classical Description • NMR Pulse Ø Applying the B 1 field for a specified duration (Pulse length or width) Ø Net Magnetization precesses about B 1 a defined angle (90 o, 180 o, etc) z z 90 o pulse Mo B 1 w 1 y x B 1 off… (or off-resonance) w 1 = g B 1 x Mxy y w 1

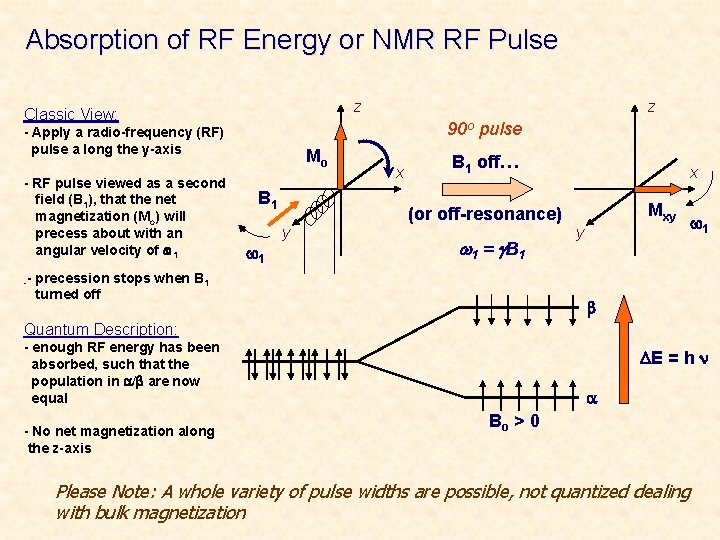
Absorption of RF Energy or NMR RF Pulse z Classic View: 90 o pulse - Apply a radio-frequency (RF) pulse a long the y-axis - RF pulse viewed as a second field (B 1), that the net magnetization (Mo) will precess about with an angular velocity of w 1 z Mo B 1 w 1 y x B 1 off… (or off-resonance) w 1 = g B 1 x Mxy y w 1 -- precession stops when B 1 turned off b Quantum Description: - enough RF energy has been absorbed, such that the population in a/b are now equal - No net magnetization along the z-axis DE = h n a Bo > 0 Please Note: A whole variety of pulse widths are possible, not quantized dealing with bulk magnetization

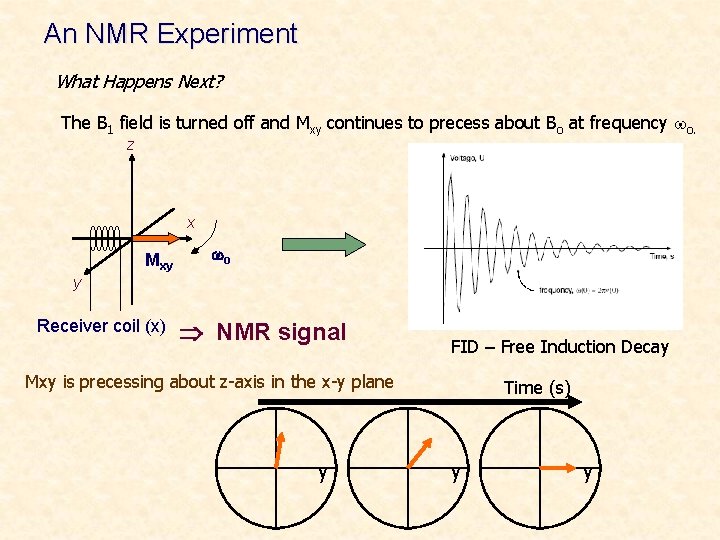
An NMR Experiment What Happens Next? The B 1 field is turned off and Mxy continues to precess about Bo at frequency wo. z x Mxy wo y Receiver coil (x) NMR signal FID – Free Induction Decay Mxy is precessing about z-axis in the x-y plane y Time (s) y y

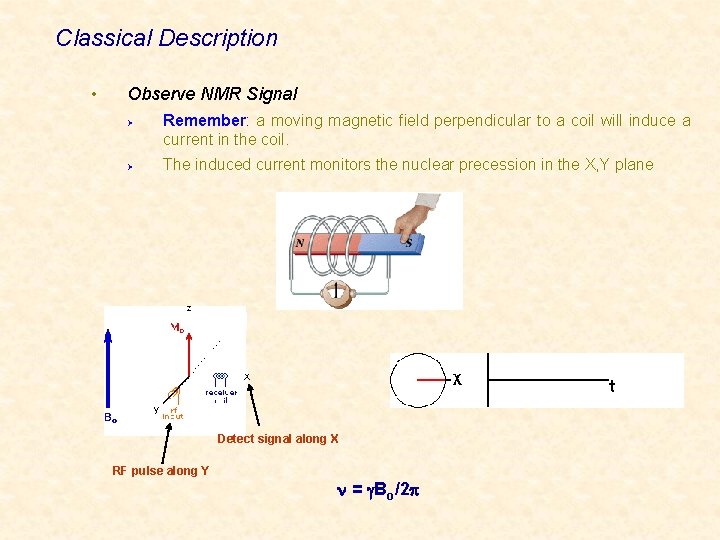
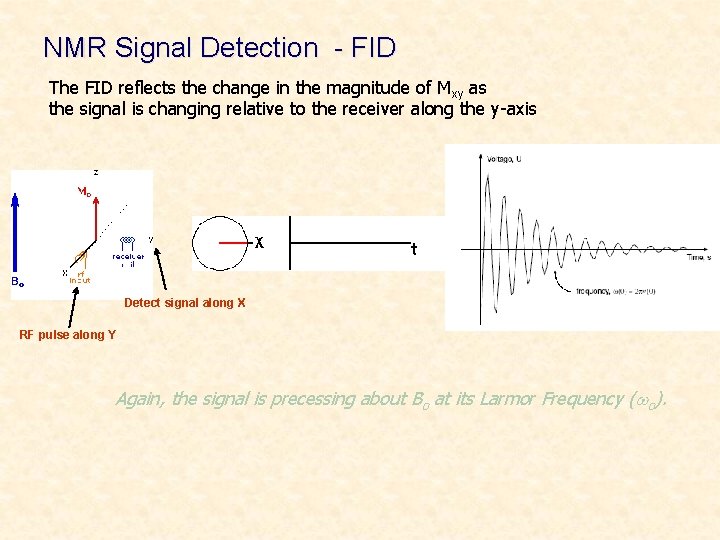
Classical Description • Observe NMR Signal Remember: a moving magnetic field perpendicular to a coil will induce a current in the coil. Ø The induced current monitors the nuclear precession in the X, Y plane Ø X y Detect signal along X RF pulse along Y n = g. Bo/2 p

NMR Signal Detection - FID The FID reflects the change in the magnitude of Mxy as the signal is changing relative to the receiver along the y-axis Detect signal along X RF pulse along Y Again, the signal is precessing about Bo at its Larmor Frequency (wo).

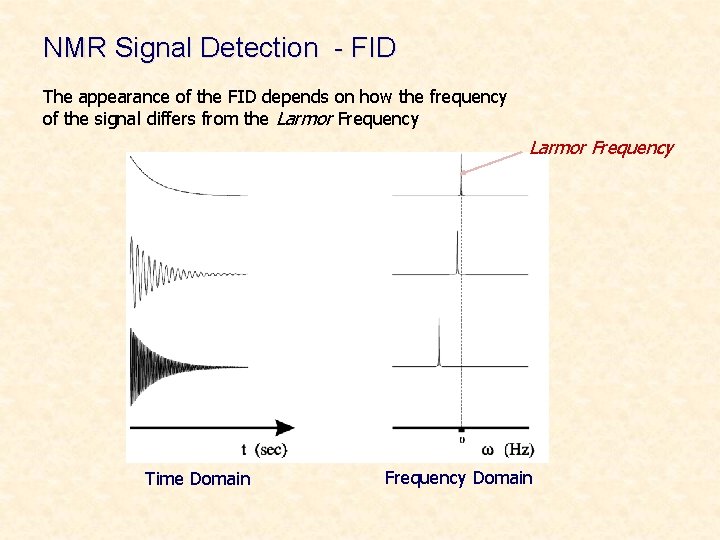
NMR Signal Detection - FID The appearance of the FID depends on how the frequency of the signal differs from the Larmor Frequency Time Domain Frequency Domain

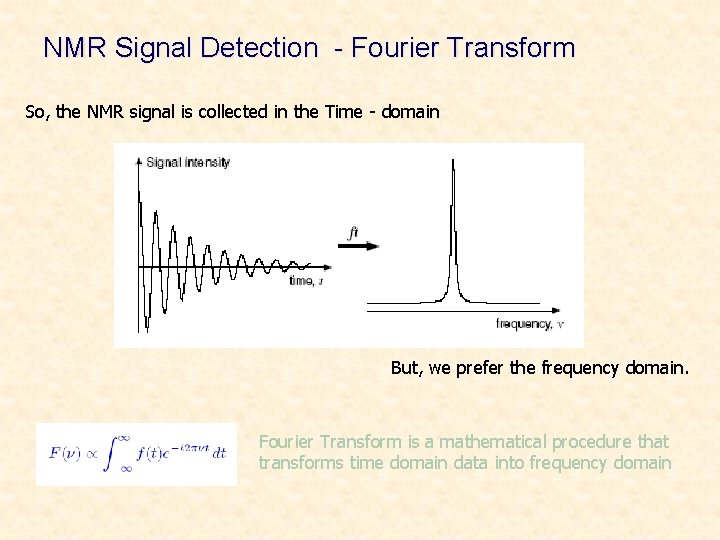
NMR Signal Detection - Fourier Transform So, the NMR signal is collected in the Time - domain But, we prefer the frequency domain. Fourier Transform is a mathematical procedure that transforms time domain data into frequency domain

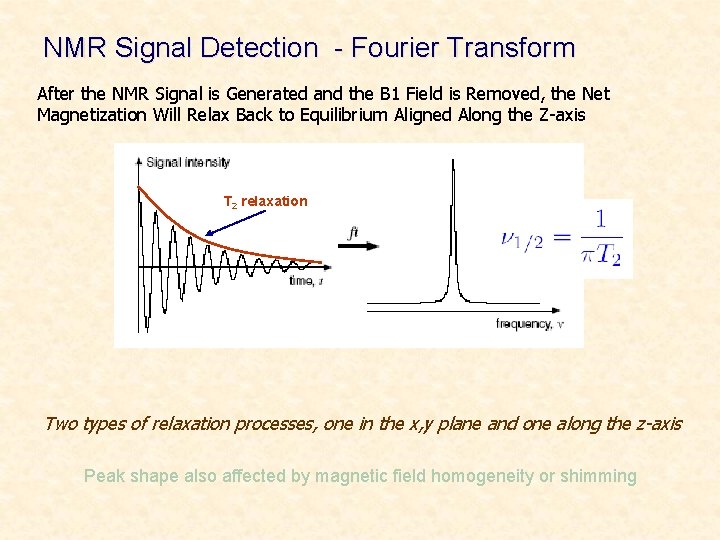
NMR Signal Detection - Fourier Transform After the NMR Signal is Generated and the B 1 Field is Removed, the Net Magnetization Will Relax Back to Equilibrium Aligned Along the Z-axis T 2 relaxation Two types of relaxation processes, one in the x, y plane and one along the z-axis Peak shape also affected by magnetic field homogeneity or shimming

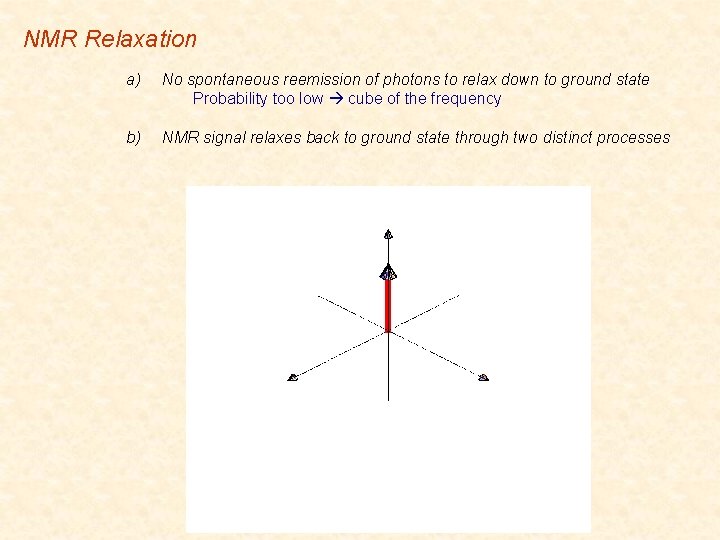
NMR Relaxation a) No spontaneous reemission of photons to relax down to ground state Probability too low cube of the frequency b) NMR signal relaxes back to ground state through two distinct processes

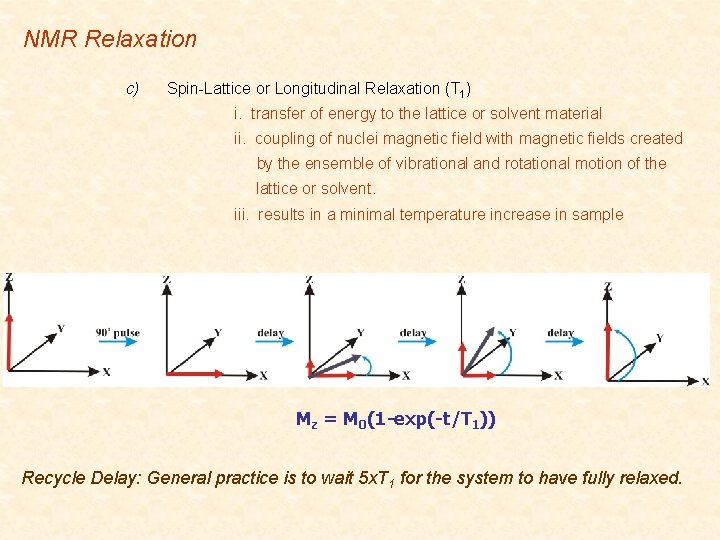
NMR Relaxation c) Spin-Lattice or Longitudinal Relaxation (T 1) i. transfer of energy to the lattice or solvent material ii. coupling of nuclei magnetic field with magnetic fields created by the ensemble of vibrational and rotational motion of the lattice or solvent. iii. results in a minimal temperature increase in sample Mz = M 0(1 -exp(-t/T 1)) Recycle Delay: General practice is to wait 5 x. T 1 for the system to have fully relaxed.

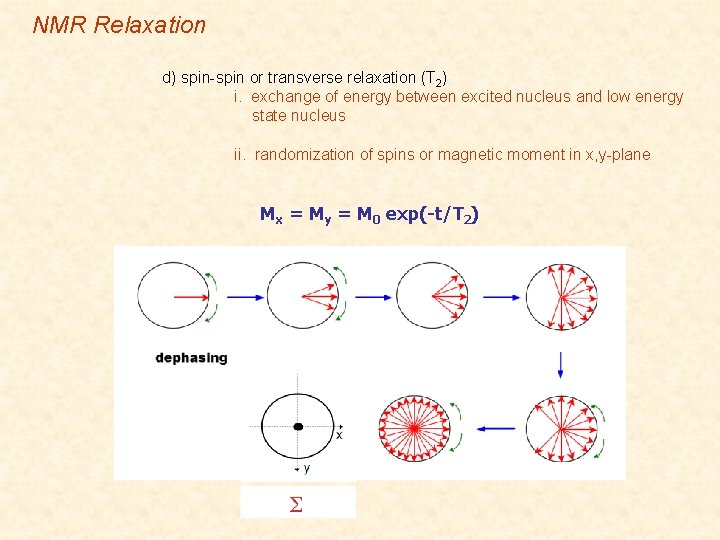
NMR Relaxation d) spin-spin or transverse relaxation (T 2) i. exchange of energy between excited nucleus and low energy state nucleus ii. randomization of spins or magnetic moment in x, y-plane Mx = My = M 0 exp(-t/T 2)

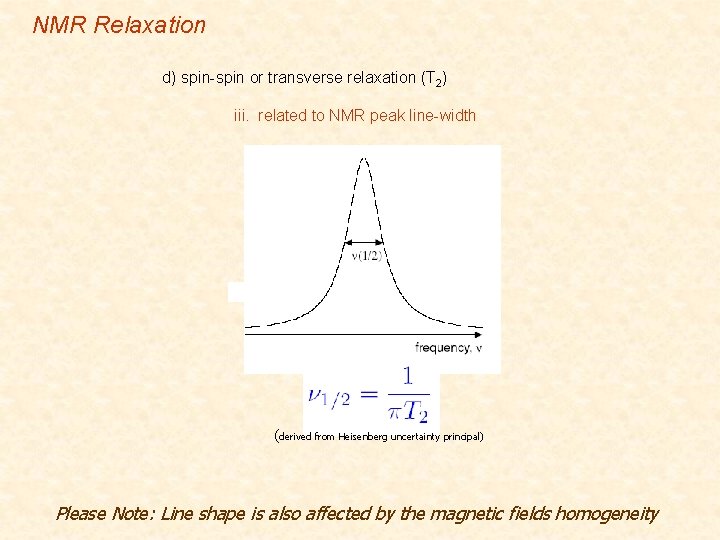
NMR Relaxation d) spin-spin or transverse relaxation (T 2) iii. related to NMR peak line-width (derived from Heisenberg uncertainty principal) Please Note: Line shape is also affected by the magnetic fields homogeneity

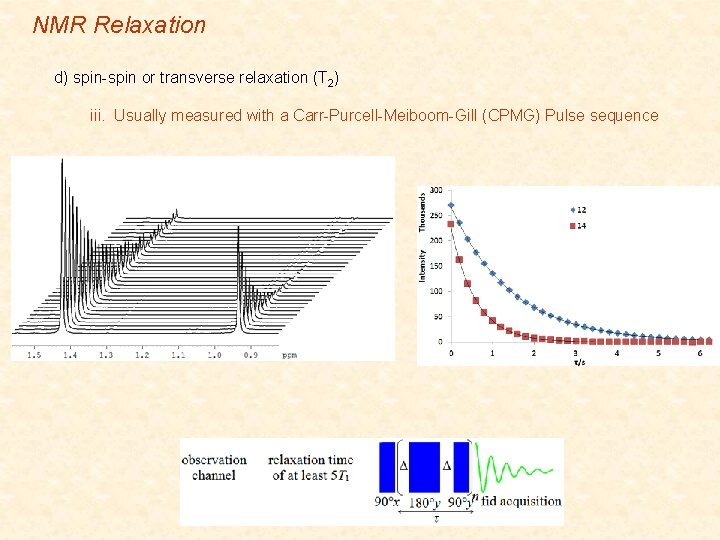
NMR Relaxation d) spin-spin or transverse relaxation (T 2) iii. Usually measured with a Carr-Purcell-Meiboom-Gill (CPMG) Pulse sequence

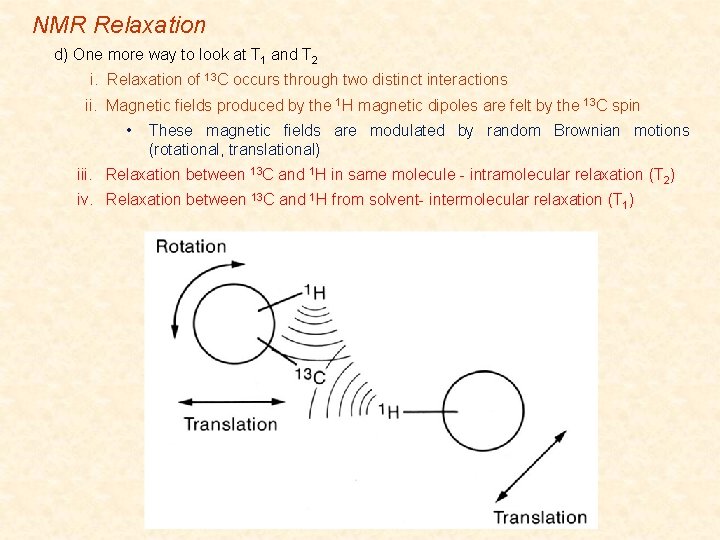
NMR Relaxation d) One more way to look at T 1 and T 2 i. Relaxation of 13 C occurs through two distinct interactions ii. Magnetic fields produced by the 1 H magnetic dipoles are felt by the 13 C spin • These magnetic fields are modulated by random Brownian motions (rotational, translational) iii. Relaxation between 13 C and 1 H in same molecule - intramolecular relaxation (T 2) iv. Relaxation between 13 C and 1 H from solvent- intermolecular relaxation (T 1)

THANKS
 Ekta khosla
Ekta khosla Ekta khosla
Ekta khosla Dept nmr spectroscopy
Dept nmr spectroscopy Cyclohexane nmr splitting
Cyclohexane nmr splitting Nmr instrumentation
Nmr instrumentation Factors influencing chemical shift
Factors influencing chemical shift Advantages of nmr spectroscopy
Advantages of nmr spectroscopy Ekta dang
Ekta dang Khosla formula for runoff
Khosla formula for runoff Aditya khosla
Aditya khosla Nityam khosla
Nityam khosla Non-dilutive funding landscape
Non-dilutive funding landscape Spectroscopy definition
Spectroscopy definition Theory of atomic absorption spectroscopy
Theory of atomic absorption spectroscopy Infrared spectroscopy theory
Infrared spectroscopy theory Multipletowość nmr
Multipletowość nmr Pola splitting nmr
Pola splitting nmr Nmr associates
Nmr associates Acorn nmr
Acorn nmr 1 bromopropane nmr
1 bromopropane nmr Larmor frequency formula
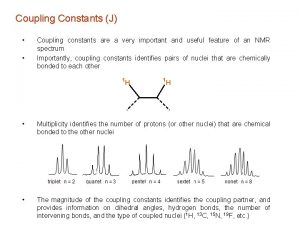
Larmor frequency formula Doublet triplet quartet
Doublet triplet quartet Shielding nmr
Shielding nmr Nmr singlet doublet triplet
Nmr singlet doublet triplet Nmr sample tube
Nmr sample tube Nmr pulse angle
Nmr pulse angle Larmor frequency
Larmor frequency Nmr lipoprofile
Nmr lipoprofile Acetylferrocene ir spectrum labeled
Acetylferrocene ir spectrum labeled Alkyne carbon nmr
Alkyne carbon nmr Nmr integration
Nmr integration Ortho meta para h nmr
Ortho meta para h nmr J'
J' Ketone nmr
Ketone nmr Hnmr
Hnmr Tabel pergeseran kimia h nmr
Tabel pergeseran kimia h nmr Nmr esr
Nmr esr Virstatin nmr
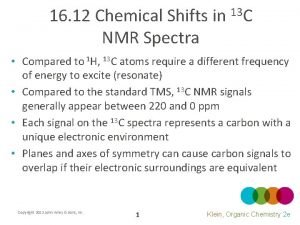
Virstatin nmr Carbon nmr shifts
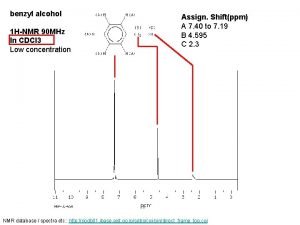
Carbon nmr shifts Benzoic acid nmr
Benzoic acid nmr