Introduction to Infra Red Spectroscopy Dr Ekta Khosla




- Slides: 14

Introduction to Infra Red Spectroscopy Dr Ekta Khosla Hans Raj Mahila Maha Vidyalaya Jalandhar

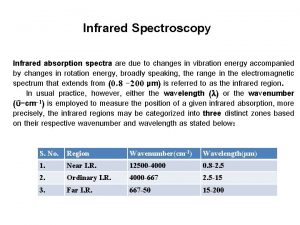
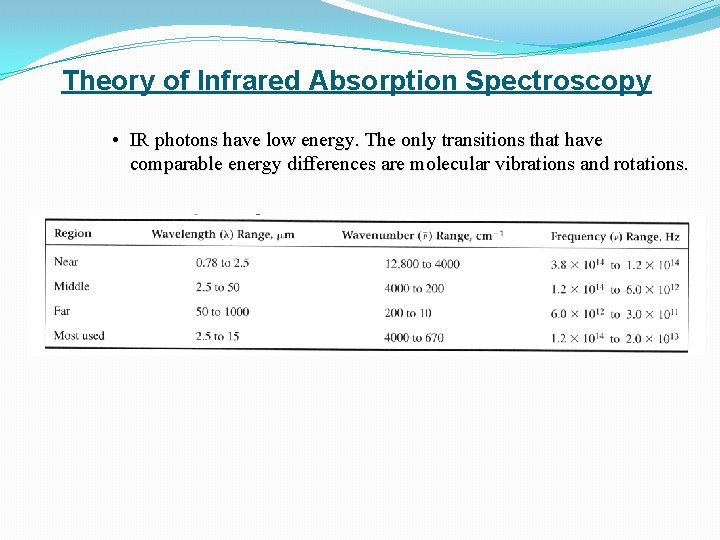
Theory of Infrared Absorption Spectroscopy • IR photons have low energy. The only transitions that have comparable energy differences are molecular vibrations and rotations.

Theory of Infrared Absorption Spectroscopy • In order for IR absorbance to occur two conditions must be met: 1. There must be a change in the dipole moment of the molecule as a result of a molecular vibration (or rotation). The change (or oscillation) in the dipole moment allows interaction with the alternating electrical component of the IR radiation wave. Symmetric molecules (or bonds) do not absorb IR radiation since there is no dipole moment. 2. If the frequency of the radiation matches the natural frequency of the vibration (or rotation), the IR photon is absorbed and the amplitude of the vibration increases.

Theory of Infrared Absorption Spectroscopy • In order for IR absorbance to occur two conditions must be met: 1. There must be a change in the dipole moment of the molecule as a result of a molecular vibration (or rotation). The change (or oscillation) in the dipole moment allows interaction with the alternating electrical component of the IR radiation wave. Symmetric molecules (or bonds) do not absorb IR radiation since there is no dipole moment. 2. If the frequency of the radiation matches the natural frequency of the vibration (or rotation), the IR photon is absorbed and the amplitude of the vibration increases.

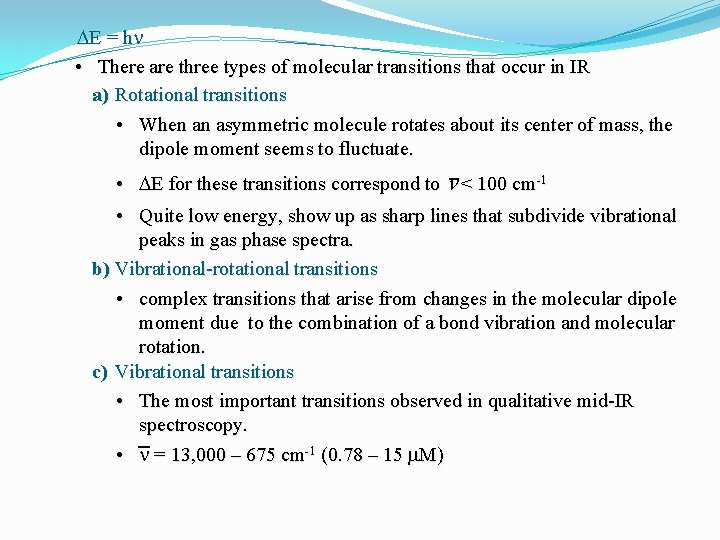
DE = h n • There are three types of molecular transitions that occur in IR a) Rotational transitions • When an asymmetric molecule rotates about its center of mass, the dipole moment seems to fluctuate. • DE for these transitions correspond to n < 100 cm-1 • Quite low energy, show up as sharp lines that subdivide vibrational peaks in gas phase spectra. b) Vibrational-rotational transitions • complex transitions that arise from changes in the molecular dipole moment due to the combination of a bond vibration and molecular rotation. c) Vibrational transitions • The most important transitions observed in qualitative mid-IR spectroscopy. • n = 13, 000 – 675 cm-1 (0. 78 – 15 m. M)

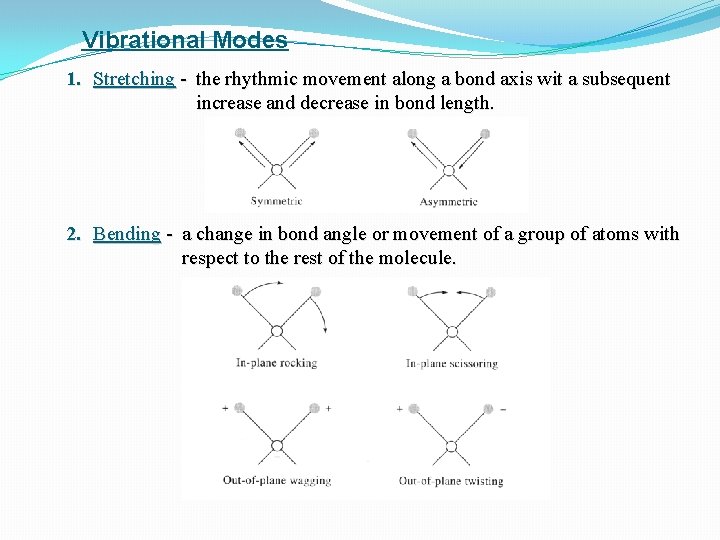
Vibrational Modes 1. Stretching - the rhythmic movement along a bond axis wit a subsequent increase and decrease in bond length. 2. Bending - a change in bond angle or movement of a group of atoms with respect to the rest of the molecule.

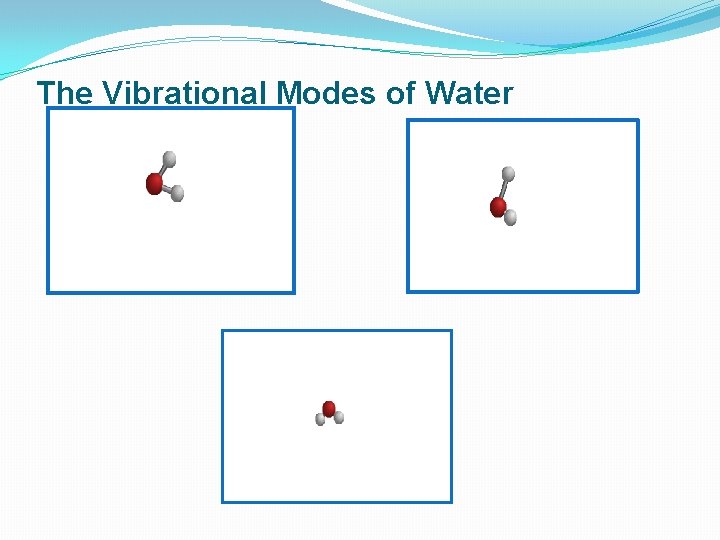
The Vibrational Modes of Water


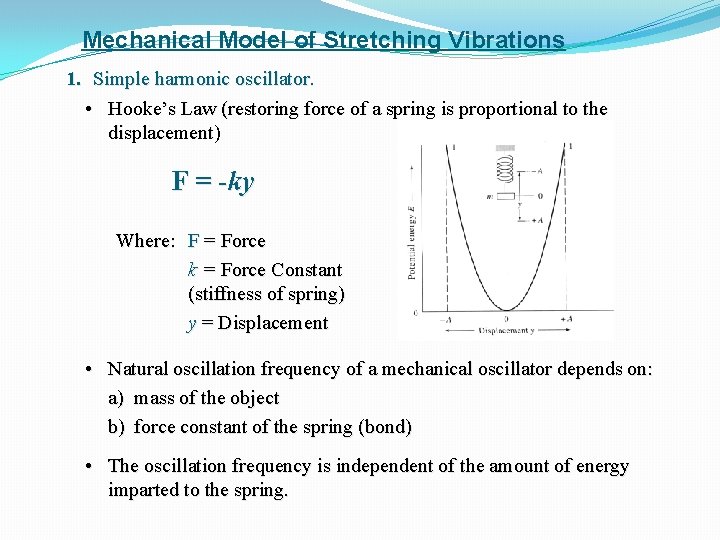
Mechanical Model of Stretching Vibrations 1. Simple harmonic oscillator. • Hooke’s Law (restoring force of a spring is proportional to the displacement) F = -ky Where: F = Force k = Force Constant (stiffness of spring) y = Displacement • Natural oscillation frequency of a mechanical oscillator depends on: a) mass of the object b) force constant of the spring (bond) • The oscillation frequency is independent of the amount of energy imparted to the spring.

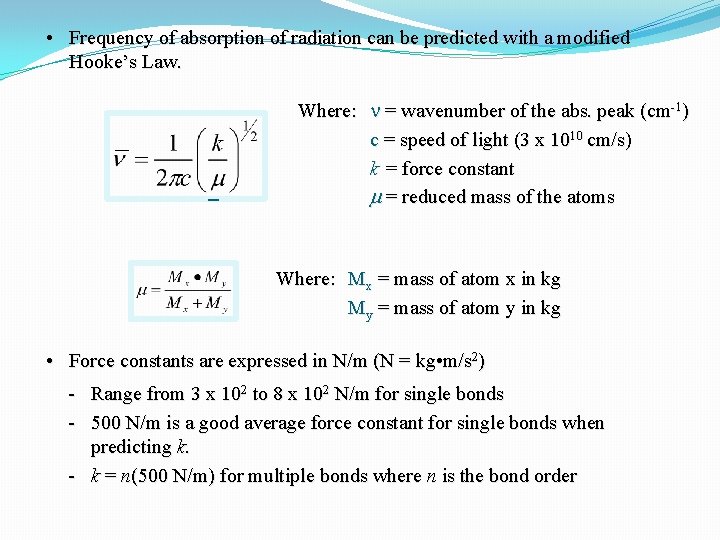
• Frequency of absorption of radiation can be predicted with a modified Hooke’s Law. Where: n = wavenumber of the abs. peak (cm-1) c = speed of light (3 x 1010 cm/s) k = force constant m = reduced mass of the atoms Where: Mx = mass of atom x in kg My = mass of atom y in kg • Force constants are expressed in N/m (N = kg • m/s 2) - Range from 3 x 102 to 8 x 102 N/m for single bonds - 500 N/m is a good average force constant for single bonds when predicting k. - k = n(500 N/m) for multiple bonds where n is the bond order

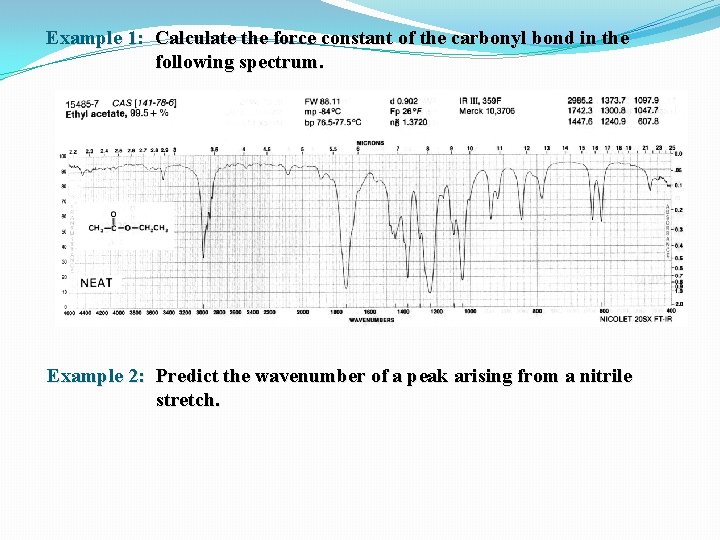
Example 1: Calculate the force constant of the carbonyl bond in the following spectrum. Example 2: Predict the wavenumber of a peak arising from a nitrile stretch.

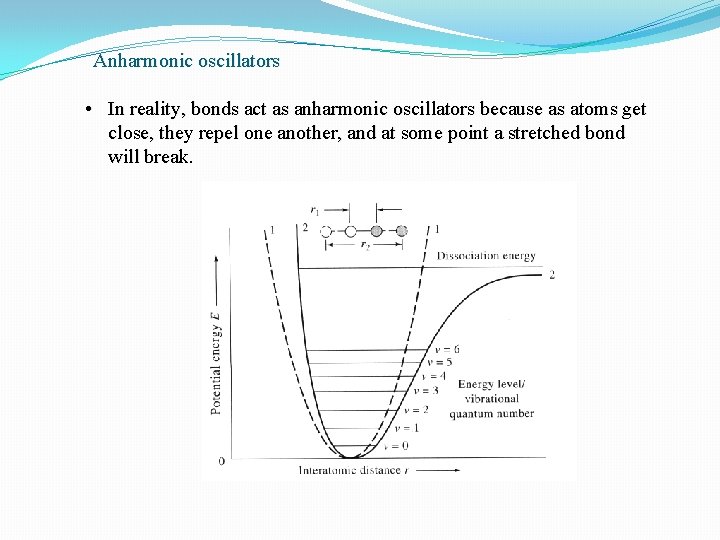
Anharmonic oscillators • In reality, bonds act as anharmonic oscillators because as atoms get close, they repel one another, and at some point a stretched bond will break.

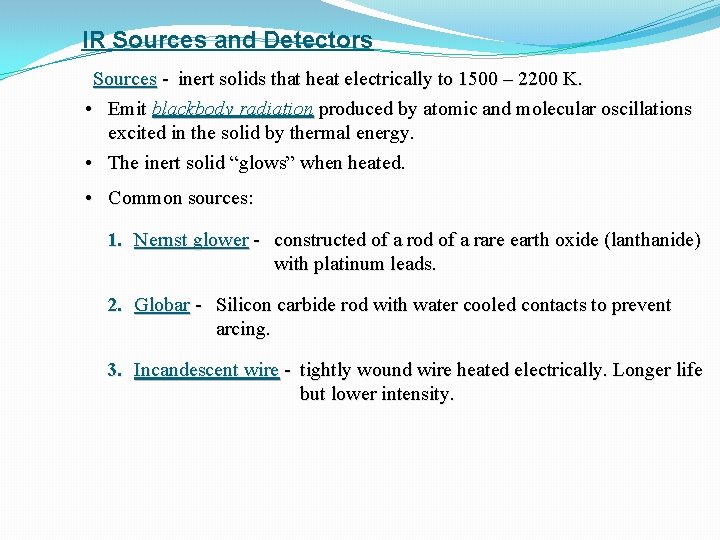
IR Sources and Detectors Sources - inert solids that heat electrically to 1500 – 2200 K. • Emit blackbody radiation produced by atomic and molecular oscillations excited in the solid by thermal energy. • The inert solid “glows” when heated. • Common sources: 1. Nernst glower - constructed of a rod of a rare earth oxide (lanthanide) with platinum leads. 2. Globar - Silicon carbide rod with water cooled contacts to prevent arcing. 3. Incandescent wire - tightly wound wire heated electrically. Longer life but lower intensity.

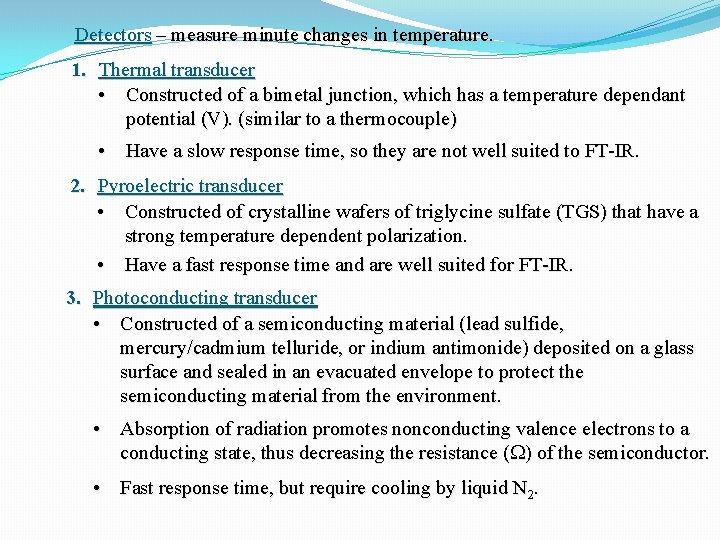
Detectors – measure minute changes in temperature. 1. Thermal transducer • Constructed of a bimetal junction, which has a temperature dependant potential (V). (similar to a thermocouple) • Have a slow response time, so they are not well suited to FT-IR. 2. Pyroelectric transducer • Constructed of crystalline wafers of triglycine sulfate (TGS) that have a strong temperature dependent polarization. • Have a fast response time and are well suited for FT-IR. 3. Photoconducting transducer • Constructed of a semiconducting material (lead sulfide, mercury/cadmium telluride, or indium antimonide) deposited on a glass surface and sealed in an evacuated envelope to protect the semiconducting material from the environment. • Absorption of radiation promotes nonconducting valence electrons to a conducting state, thus decreasing the resistance (W) of the semiconductor. • Fast response time, but require cooling by liquid N 2.
 Ekta khosla
Ekta khosla Ekta khosla
Ekta khosla Ekta dang
Ekta dang Infra red
Infra red Khosla formula for runoff
Khosla formula for runoff Aditya khosla
Aditya khosla Cargo community systems
Cargo community systems Non dilutive funding landscape
Non dilutive funding landscape Introduction to molecular spectroscopy
Introduction to molecular spectroscopy Uv visible spectroscopy introduction
Uv visible spectroscopy introduction Cellule de haller
Cellule de haller Glândulas de schietzel
Glândulas de schietzel Infra ry
Infra ry Infra auricular region
Infra auricular region Boundaries of infratemporal fossa
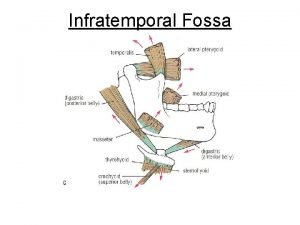
Boundaries of infratemporal fossa