The impact of dolutegravir in firstline adult ART

- Slides: 22

The impact of dolutegravir in firstline adult ART on HIV transmission and cost of HIV in South Africa Sithabiso Masuku 1, Gesine Meyer-Rath 1, 2, Lise Jamieson 1, Francois Venter 3, Leigh Johnson 4 1 Health Economics and Epidemiology Research Office, University of the Witwatersrand, South Africa 2 Center for Global Health and Development, Boston University, US 3 Wits Reproductive Health and HIV Unit, University of the Witwatersrand, Johannesburg, South Africa 4 Centre for Infectious Disease Epidemiology and Research, University of Cape Town, South Africa International AIDS Economics Network (IAEN) Conference 20 July 2018

Background South Africa is considering switching all adults on first-line ART regimens containing efavirenz (EFV) to regimens containing dolutegravir (DTG) tenofovir + emtricitabine + efavirenz (TEE) We examined the impact of switching on: § HIV transmission § AIDS mortality § cost of the HIV programme tenofovir + lamivudine + dolutegravir (TLD)

Scenarios and treatment populations 1. Current pace of scale-up of universal test and treat (UTT) (40% of newly diagnosed PLHIV initiate ART within a year) A. TEE (Baseline) B. TLD for all adults C. TLD for men only D. TLD for men and women >55 2. Rapid UTT scale-up (95% of newly diagnosed PLHIV initiate ART within a year) • Cost and impact of all scenarios analysed incrementally to a baseline of TEE at current pace of UTT scale-up

Epidemiological model • Epidemiological impact was analysed using Thembisa, a compartmental HIV transmission model fitted to the South African HIV epidemic • Updates to ART assumptions made in Thembisa: § TLD increases viral suppression by 8. 3%, from 75. 6% to 83. 9% § No treatment failure under TLD

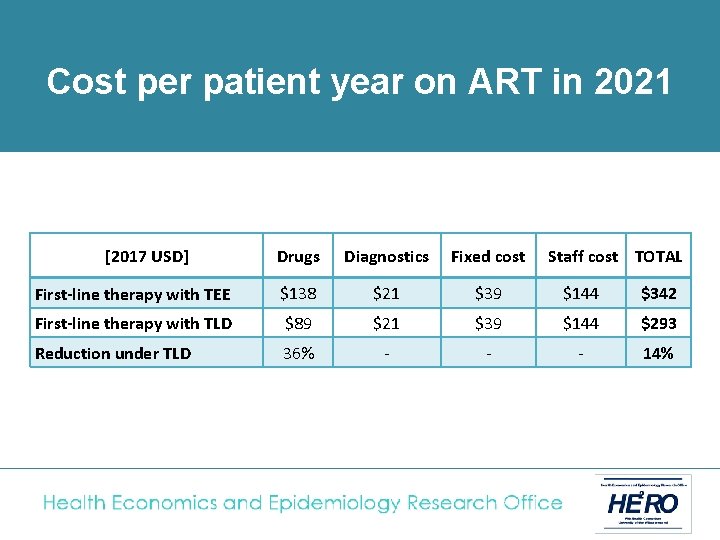
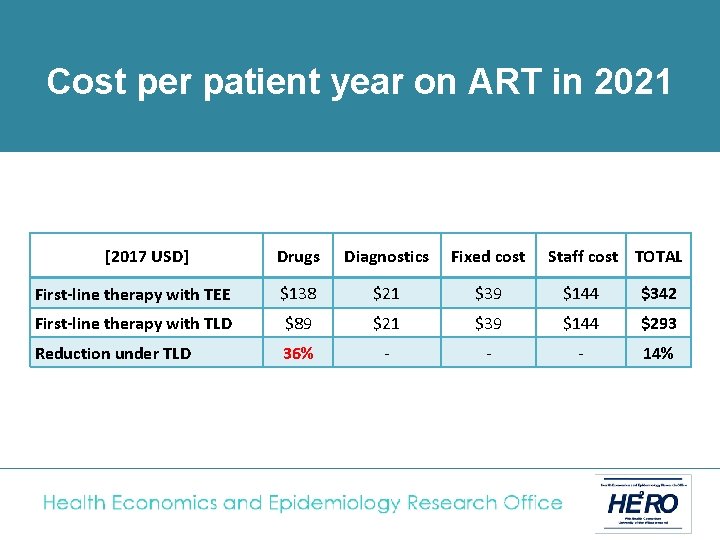
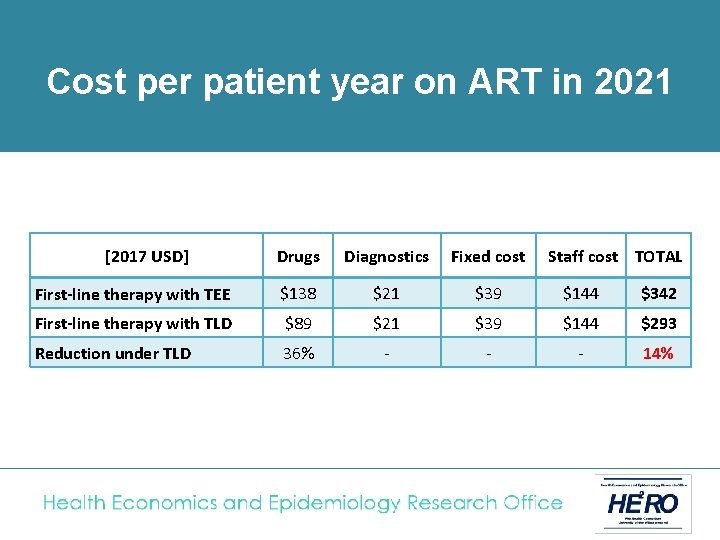
Cost analysis • The National ART Cost Model (NACM) was used to calculate the average cost per adult of TEE or TLD fixed-dose combination regimens • Adult patients on first-line treatment switched to TLD in April 2019, with full roll-out to all existing and new first-line patients by April 2020. • TLD price based on recent negotiations ($75 per patient year); same laboratory monitoring cost as TEE (1 creatinine test/ year) • Costs analysed from South African government perspective and presented in 2017 USD

of treatment in 2021 Cost. Unit percost patient year on ART in 2021 [2017 USD] Drugs Diagnostics Fixed cost Staff cost TOTAL First-line therapy with TEE $138 $21 $39 $144 $342 First-line therapy with TLD $89 $21 $39 $144 $293 Reduction under TLD 36% - - - 14%

of treatment in 2021 Cost. Unit percost patient year on ART in 2021 [2017 USD] Drugs Diagnostics Fixed cost Staff cost TOTAL First-line therapy with TEE $138 $21 $39 $144 $342 First-line therapy with TLD $89 $21 $39 $144 $293 Reduction under TLD 36% - - - 14%

of treatment in 2021 Cost. Unit percost patient year on ART in 2021 [2017 USD] Drugs Diagnostics Fixed cost Staff cost TOTAL First-line therapy with TEE $138 $21 $39 $144 $342 First-line therapy with TLD $89 $21 $39 $144 $293 Reduction under TLD 36% - - - 14%

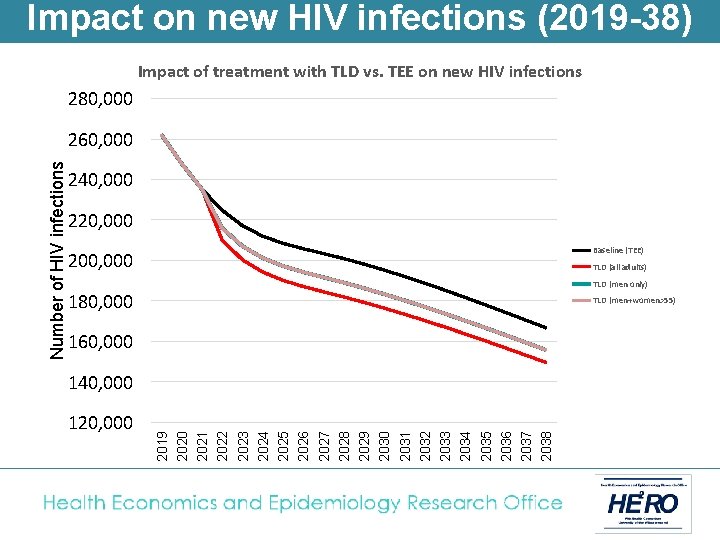
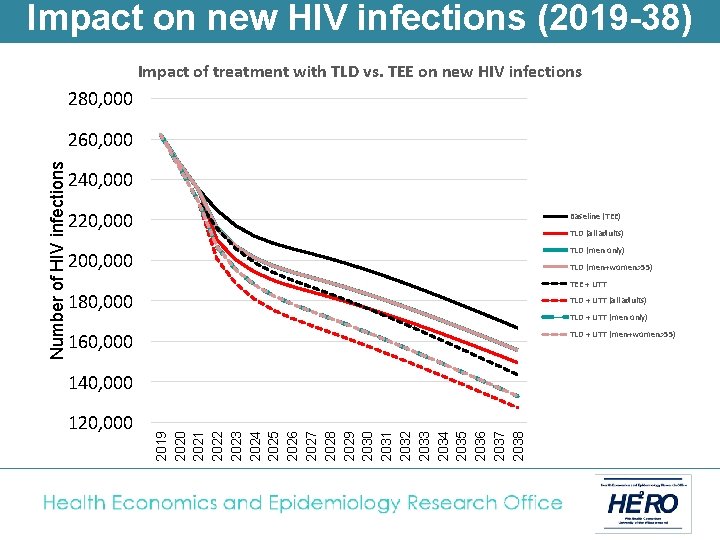
Impact on new HIV infections (2019 -38) Impact of treatment with TLD vs. TEE on new HIV infections 280, 000 240, 000 220, 000 Baseline (TEE) 200, 000 TLD (all adults) TLD (men only) 180, 000 TLD (men+women>55) 160, 000 140, 000 120, 000 2019 2020 2021 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 Number of HIV infections 260, 000

Impact on new HIV infections (2019 -38) Impact of treatment with TLD vs. TEE on new HIV infections 280, 000 240, 000 220, 000 Baseline (TEE) 200, 000 TLD (men only) TLD (all adults) TLD (men+women>55) TEE + UTT 180, 000 TLD + UTT (all adults) TLD + UTT (men only) TLD + UTT (men+women>55) 160, 000 140, 000 120, 000 2019 2020 2021 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 Number of HIV infections 260, 000

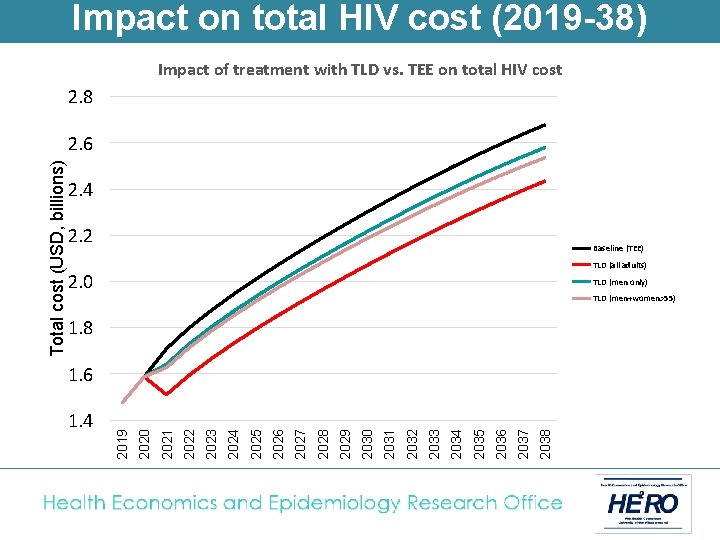
Impact on total HIV cost (2019 -38) Impact of treatment with TLD vs. TEE on total HIV cost 2. 8 2. 4 2. 2 Baseline (TEE) TLD (all adults) 2. 0 TLD (men only) TLD (men+women>55) 1. 8 2037 2036 2035 2034 2033 2032 2031 2030 2029 2028 2027 2026 2025 2024 2023 2022 2021 1. 4 2020 1. 6 2019 Total cost (USD, billions) 2. 6

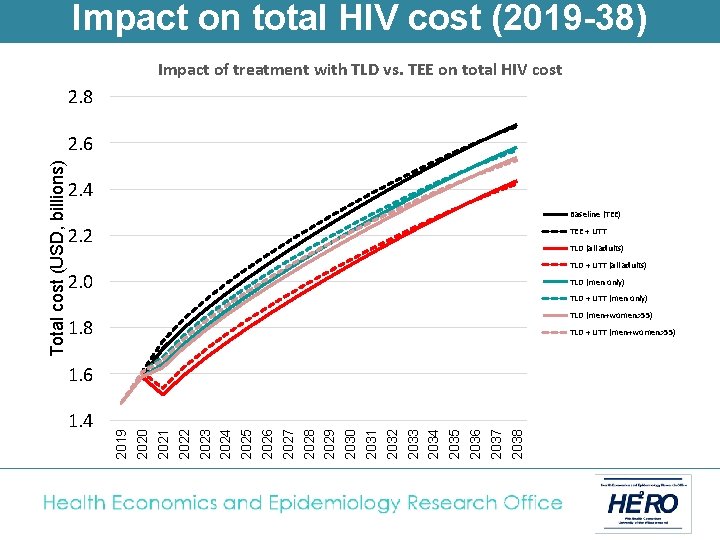
Impact on total HIV cost (2019 -38) Impact of treatment with TLD vs. TEE on total HIV cost 2. 8 2. 4 Baseline (TEE) 2. 2 TEE + UTT TLD (all adults) TLD + UTT (all adults) 2. 0 TLD (men only) TLD + UTT (men only) TLD (men+women>55) 1. 8 TLD + UTT (men+women>55) 1. 6 1. 4 2019 2020 2021 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 Total cost (USD, billions) 2. 6

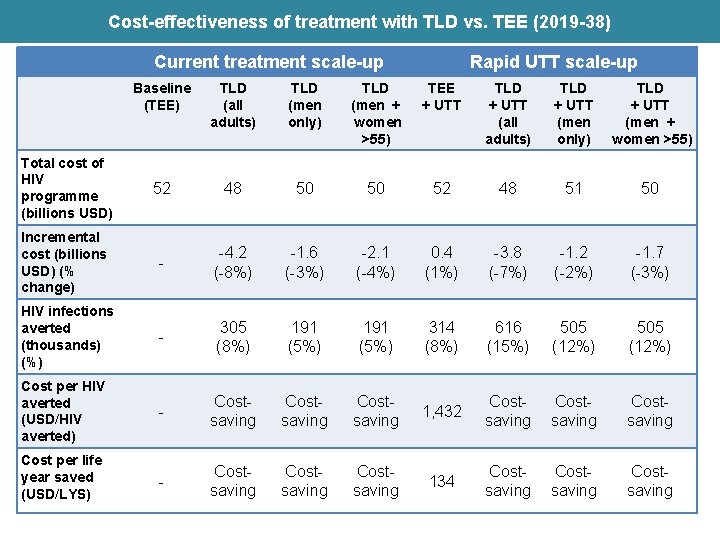
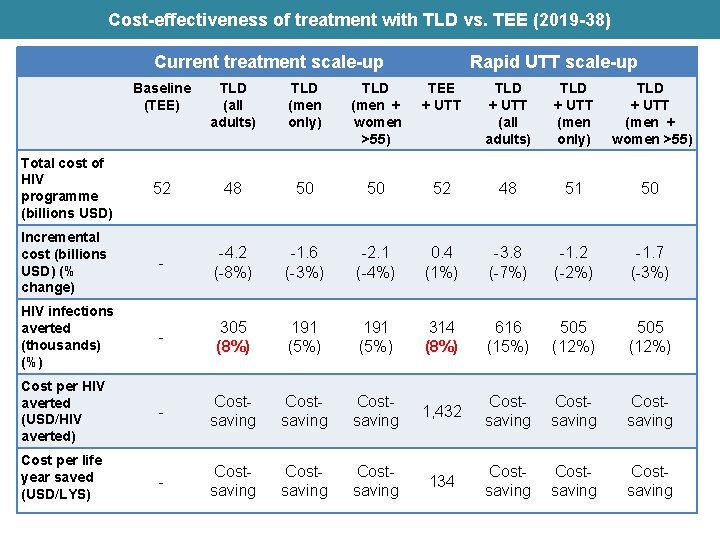
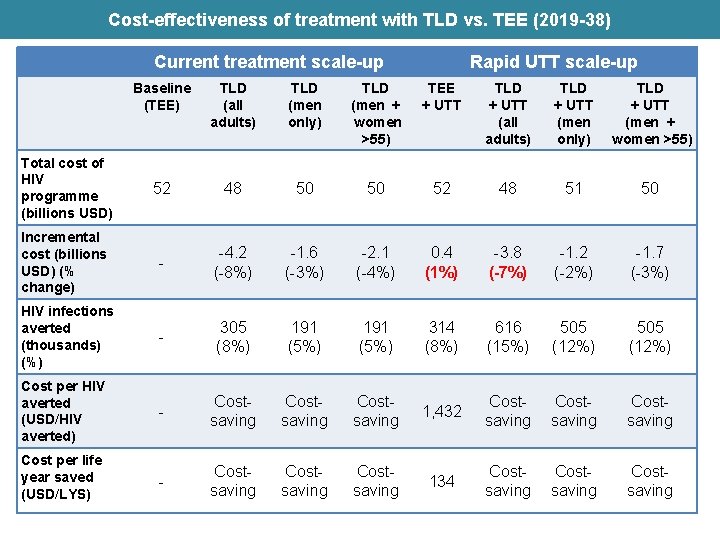
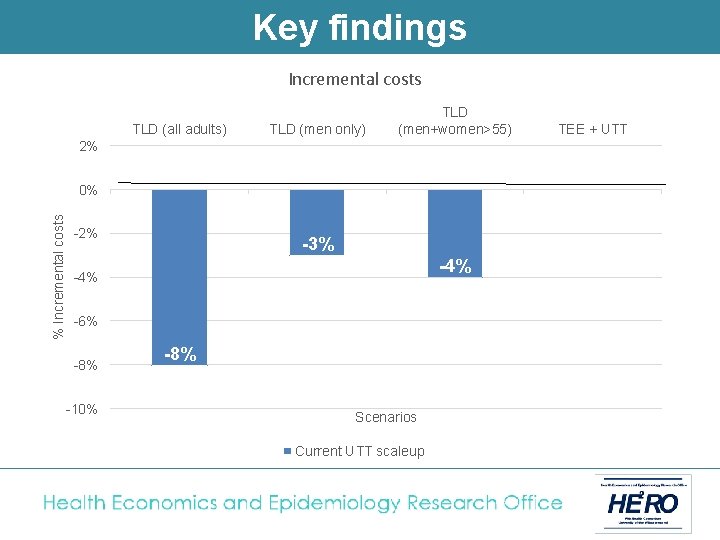
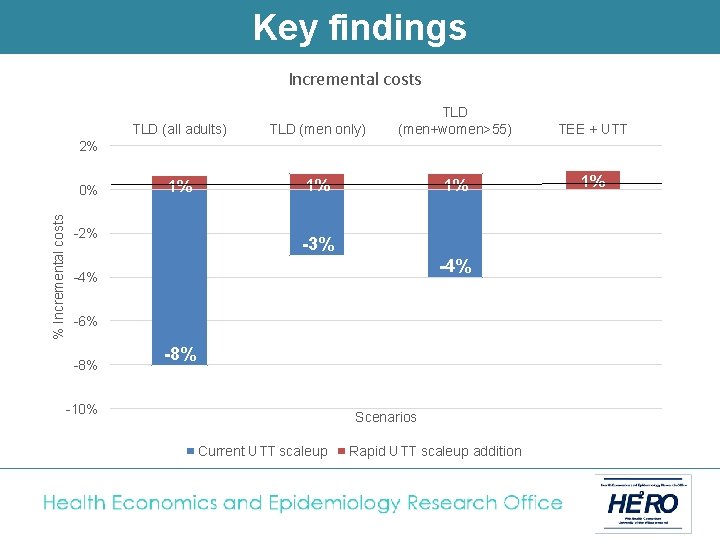
Cost-effectiveness of treatment with TLD vs. TEE (2019 -38) Current treatment scale-up Rapid UTT scale-up Baseline (TEE) TLD (all adults) TLD (men only) TLD (men + women >55) TEE + UTT TLD + UTT (all adults) TLD + UTT (men only) TLD + UTT (men + women >55) Total cost of HIV programme (billions USD) 52 48 50 50 52 48 51 50 Incremental cost (billions USD) (% change) - -4. 2 (-8%) -1. 6 (-3%) -2. 1 (-4%) 0. 4 (1%) -3. 8 (-7%) -1. 2 (-2%) -1. 7 (-3%) HIV infections averted (thousands) (%) - 305 (8%) 191 (5%) 314 (8%) 616 (15%) 505 (12%) Cost per HIV averted (USD/HIV averted) - Costsaving 1, 432 Costsaving Cost per life year saved (USD/LYS) - Costsaving 134 Costsaving

Cost-effectiveness of treatment with TLD vs. TEE (2019 -38) Current treatment scale-up Rapid UTT scale-up Baseline (TEE) TLD (all adults) TLD (men only) TLD (men + women >55) TEE + UTT TLD + UTT (all adults) TLD + UTT (men only) TLD + UTT (men + women >55) Total cost of HIV programme (billions USD) 52 48 50 50 52 48 51 50 Incremental cost (billions USD) (% change) - -4. 2 (-8%) -1. 6 (-3%) -2. 1 (-4%) 0. 4 (1%) -3. 8 (-7%) -1. 2 (-2%) -1. 7 (-3%) HIV infections averted (thousands) (%) - 305 (8%) 191 (5%) 314 (8%) 616 (15%) 505 (12%) Cost per HIV averted (USD/HIV averted) - Costsaving 1, 432 Costsaving Cost per life year saved (USD/LYS) - Costsaving 134 Costsaving

Cost-effectiveness of treatment with TLD vs. TEE (2019 -38) Current treatment scale-up Rapid UTT scale-up Baseline (TEE) TLD (all adults) TLD (men only) TLD (men + women >55) TEE + UTT TLD + UTT (all adults) TLD + UTT (men only) TLD + UTT (men + women >55) Total cost of HIV programme (billions USD) 52 48 50 50 52 48 51 50 Incremental cost (billions USD) (% change) - -4. 2 (-8%) -1. 6 (-3%) -2. 1 (-4%) 0. 4 (1%) -3. 8 (-7%) -1. 2 (-2%) -1. 7 (-3%) HIV infections averted (thousands) (%) - 305 (8%) 191 (5%) 314 (8%) 616 (15%) 505 (12%) Cost per HIV averted (USD/HIV averted) - Costsaving 1, 432 Costsaving Cost per life year saved (USD/LYS) - Costsaving 134 Costsaving

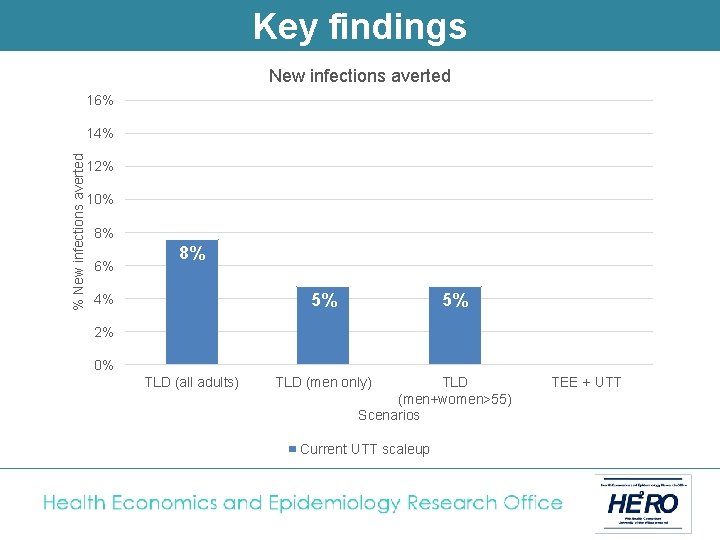
Key findings New infections averted 16% % New infections averted 14% 12% 10% 8% 6% 8% 5% 4% 5% 2% 0% TLD (all adults) TLD (men only) TLD (men+women>55) Scenarios Current UTT scaleup TEE + UTT

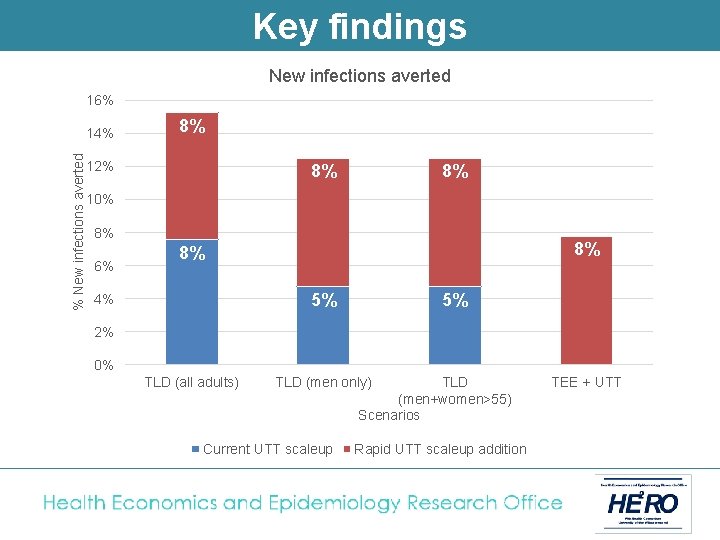
Key findings New infections averted 16% % New infections averted 14% 8% 12% 8% 8% 10% 8% 6% 8% 8% 5% 4% 5% 2% 0% TLD (all adults) TLD (men only) Current UTT scaleup TLD (men+women>55) Scenarios Rapid UTT scaleup addition TEE + UTT

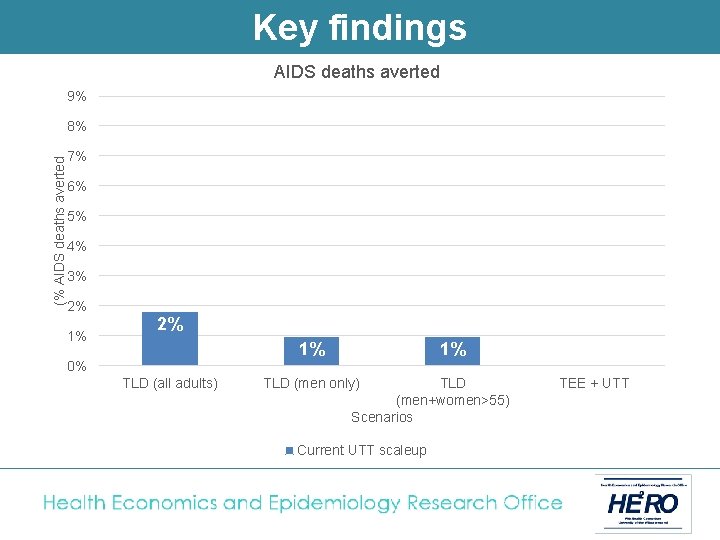
Key findings AIDS deaths averted 9% (% AIDS deaths averted 8% 7% 6% 5% 4% 3% 2% 1% 0% TLD (all adults) 1% TLD (men only) TLD (men+women>55) Scenarios Current UTT scaleup TEE + UTT

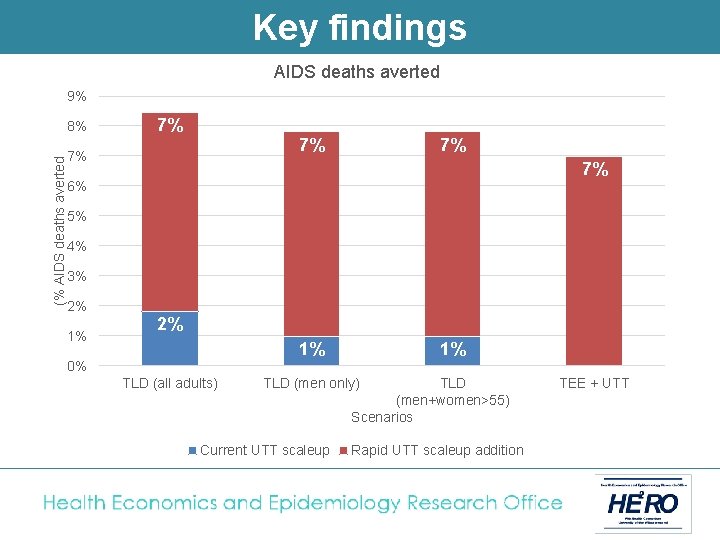
Key findings AIDS deaths averted 9% (% AIDS deaths averted 8% 7% 7% 7% 6% 5% 4% 3% 2% 1% 0% TLD (all adults) 1% TLD (men only) Current UTT scaleup TLD (men+women>55) Scenarios Rapid UTT scaleup addition TEE + UTT

Key findings Incremental costs TLD (all adults) TLD (men only) TLD (men+women>55) 2% % Incremental costs 0% -2% -3% -4% -6% -8% -10% -8% Scenarios Current UTT scaleup TEE + UTT

Key findings Incremental costs TLD (all adults) TLD (men only) TLD (men+women>55) 1% 1% 1% TEE + UTT 1% 2% % Incremental costs 0% -2% -3% -4% -6% -8% -10% Scenarios Current UTT scaleup Rapid UTT scaleup addition

Conclusion Switching adults from TEE to TLD and fully implementing UTT results in the following: • A reduction of at least 5% in new HIV infections, and 1 -2% in AIDS deaths • A reduction in the cost of South Africa’s HIV programme of between 2 -8% § Lower drug cost per patient year § Less need for second line § Less new infections • TLD to men + women outside child-bearing age is superior to TLD for men only. • TLD to all adults (with added contraception coverage for women) is the most likely policy under discussion in South Africa at the moment.
 365f1
365f1 Dolutegravir lamivudina tenofovir disoproxil fumarate
Dolutegravir lamivudina tenofovir disoproxil fumarate Dolutegravir lamivudine
Dolutegravir lamivudine Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn










































