Dolutegravir in Patients with IntegraseResistant HIV VIKING4 Dolutegravir



- Slides: 10

Dolutegravir in Patients with Integrase-Resistant HIV VIKING-4

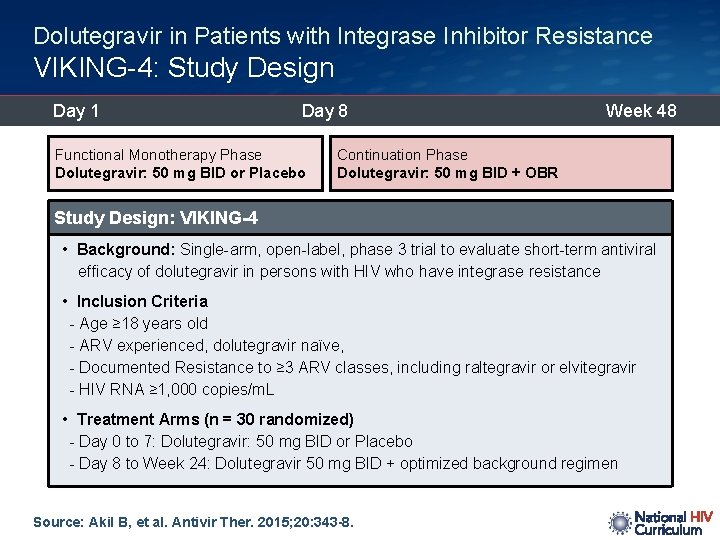
Dolutegravir in Patients with Integrase Inhibitor Resistance VIKING-4: Study Design Day 1 Day 8 Week 48 Randomize Functional Monotherapy Phase Dolutegravir: 50 mg BID or Placebo Continuation Phase Dolutegravir: 50 mg BID + OBR Study Design: VIKING-4 • Background: Single-arm, open-label, phase 3 trial to evaluate short-term antiviral efficacy of dolutegravir in persons with HIV who have integrase resistance • Inclusion Criteria - Age ≥ 18 years old - ARV experienced, dolutegravir naïve, - Documented Resistance to ≥ 3 ARV classes, including raltegravir or elvitegravir - HIV RNA ≥ 1, 000 copies/m. L • Treatment Arms (n = 30 randomized) - Day 0 to 7: Dolutegravir: 50 mg BID or Placebo - Day 8 to Week 24: Dolutegravir 50 mg BID + optimized background regimen Source: Akil B, et al. Antivir Ther. 2015; 20: 343 -8.

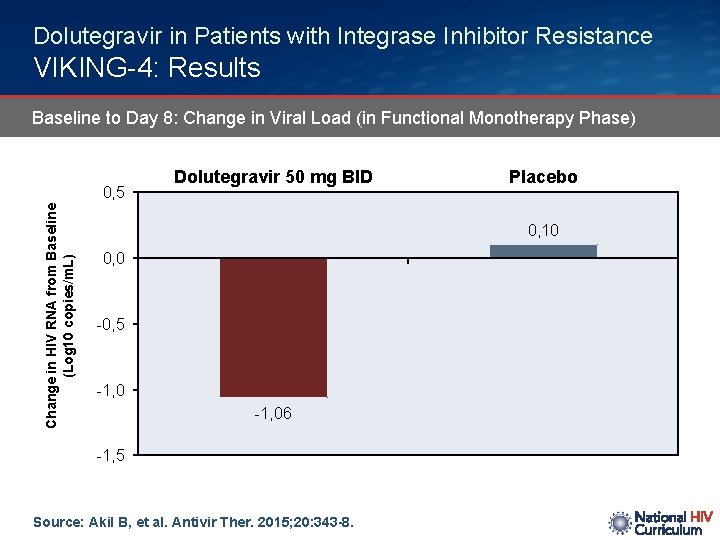
Dolutegravir in Patients with Integrase Inhibitor Resistance VIKING-4: Results Baseline to Day 8: Change in Viral Load (in Functional Monotherapy Phase) Change in HIV RNA from Baseline (Log 10 copies/m. L) 0, 5 Dolutegravir 50 mg BID Placebo 0, 10 0, 0 -0, 5 -1, 06 -1, 5 Source: Akil B, et al. Antivir Ther. 2015; 20: 343 -8.

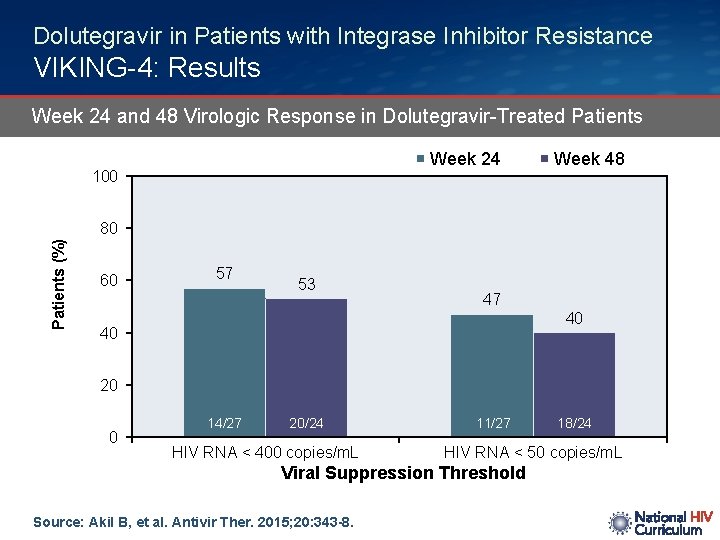
Dolutegravir in Patients with Integrase Inhibitor Resistance VIKING-4: Results Week 24 and 48 Virologic Response in Dolutegravir-Treated Patients Week 24 100 Week 48 Patients (%) 80 60 57 53 47 40 40 20 0 14/27 20/24 HIV RNA < 400 copies/m. L 11/27 HIV RNA < 50 copies/m. L Viral Suppression Threshold Source: Akil B, et al. Antivir Ther. 2015; 20: 343 -8. 18/24

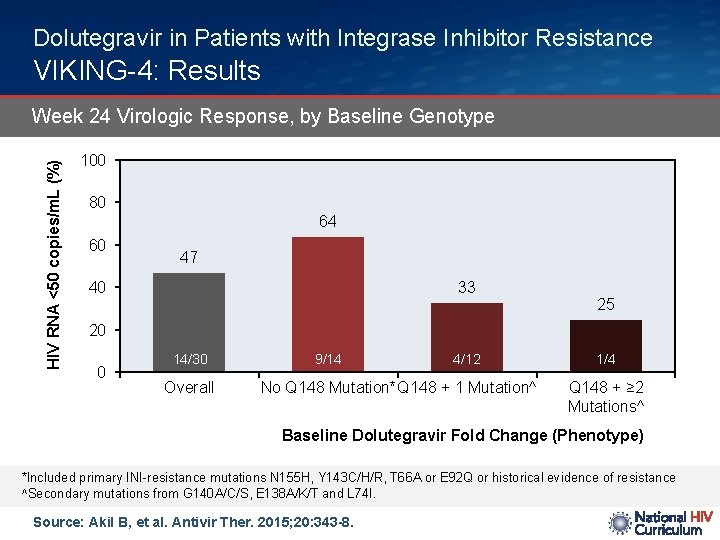
Dolutegravir in Patients with Integrase Inhibitor Resistance VIKING-4: Results HIV RNA <50 copies/m. L (%) Week 24 Virologic Response, by Baseline Genotype 100 80 64 60 47 33 40 25 20 0 14/30 Overall 9/14 4/12 No Q 148 Mutation*Q 148 + 1 Mutation^ 1/4 Q 148 + ≥ 2 Mutations^ Baseline Dolutegravir Fold Change (Phenotype) *Included primary INI-resistance mutations N 155 H, Y 143 C/H/R, T 66 A or E 92 Q or historical evidence of resistance ^Secondary mutations from G 140 A/C/S, E 138 A/K/T and L 74 I. Source: Akil B, et al. Antivir Ther. 2015; 20: 343 -8.

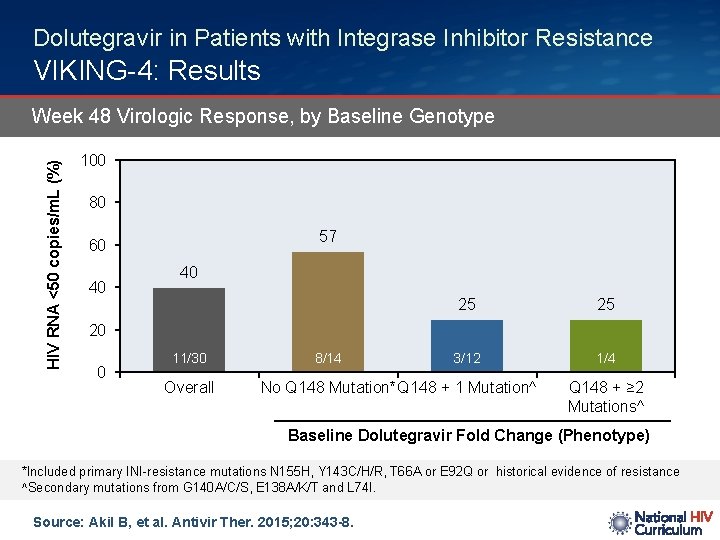
Dolutegravir in Patients with Integrase Inhibitor Resistance VIKING-4: Results HIV RNA <50 copies/m. L (%) Week 48 Virologic Response, by Baseline Genotype 100 80 57 60 40 40 25 25 3/12 1/4 20 0 11/30 Overall 8/14 No Q 148 Mutation*Q 148 + 1 Mutation^ Q 148 + ≥ 2 Mutations^ Baseline Dolutegravir Fold Change (Phenotype) *Included primary INI-resistance mutations N 155 H, Y 143 C/H/R, T 66 A or E 92 Q or historical evidence of resistance ^Secondary mutations from G 140 A/C/S, E 138 A/K/T and L 74 I. Source: Akil B, et al. Antivir Ther. 2015; 20: 343 -8.

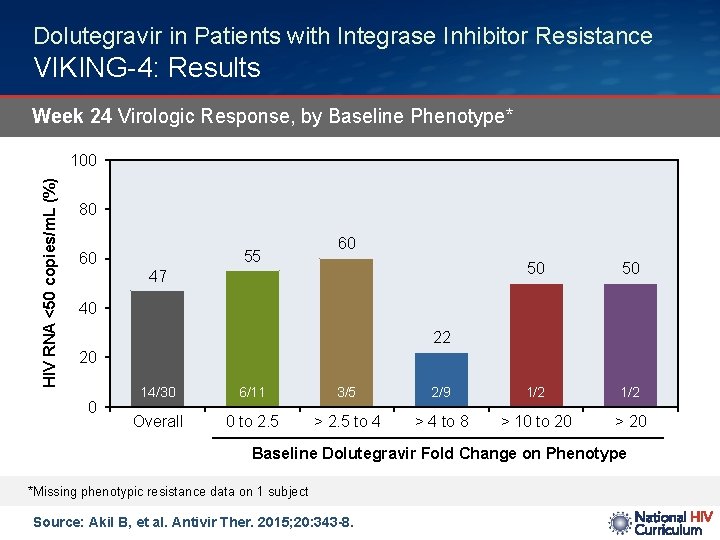
Dolutegravir in Patients with Integrase Inhibitor Resistance VIKING-4: Results Week 24 Virologic Response, by Baseline Phenotype* HIV RNA <50 copies/m. L (%) 100 80 60 55 60 47 50 50 40 22 20 0 14/30 6/11 3/5 2/9 1/2 Overall 0 to 2. 5 > 2. 5 to 4 > 4 to 8 > 10 to 20 > 20 Baseline Dolutegravir Fold Change on Phenotype *Missing phenotypic resistance data on 1 subject Source: Akil B, et al. Antivir Ther. 2015; 20: 343 -8.

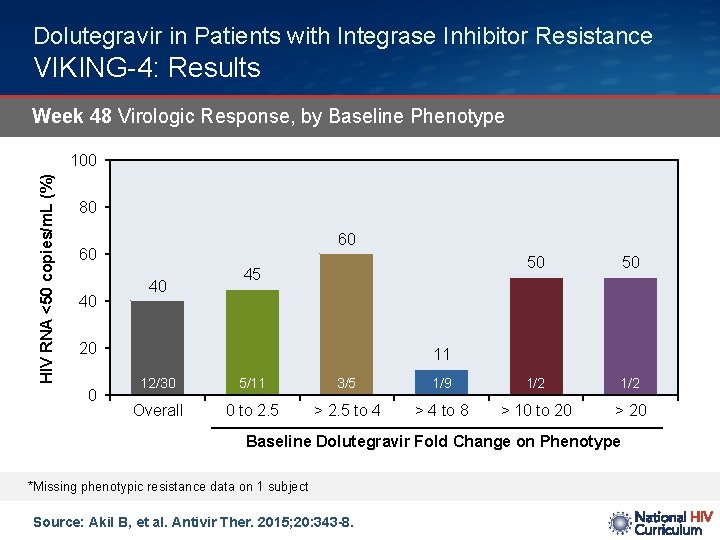
Dolutegravir in Patients with Integrase Inhibitor Resistance VIKING-4: Results Week 48 Virologic Response, by Baseline Phenotype HIV RNA <50 copies/m. L (%) 100 80 60 60 40 40 45 20 0 50 50 11 12/30 5/11 3/5 1/9 1/2 Overall 0 to 2. 5 > 2. 5 to 4 > 4 to 8 > 10 to 20 > 20 Baseline Dolutegravir Fold Change on Phenotype *Missing phenotypic resistance data on 1 subject Source: Akil B, et al. Antivir Ther. 2015; 20: 343 -8.

Dolutegravir in Patients with Raltegravir Resistance VIKING-4: Conclusions: “The observed day 8 antiviral activity in this highly treatment-experienced population with INI-resistant HIV-1 was attributable to dolutegravir. Longer-term efficacy (after considering baseline ART resistance) and safety during the open-label phase were in -line with the results of the larger VIKING-3 study. ” Source: Akil B, et al. Antivir Ther. 2015; 20: 343 -8.

Acknowledgment The National HIV Curriculum is an AIDS Education and Training Center (AETC) Program supported by the Health Resources and Services Administration (HRSA) of the U. S. Department of Health and Human Services (HHS) as part of an award totaling $800, 000 with 0% financed with non-governmental sources. This project is led by the University of Washington’s Infectious Diseases Education and Assessment (IDEA) Program. The content in this presentation are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U. S. Government.