The Epidemiology of Head and Neck Cancer Scott



























- Slides: 54

The Epidemiology of Head and Neck Cancer Scott Langevin, MHA CT(ASCP) Doctoral Candidate June 5, 2009

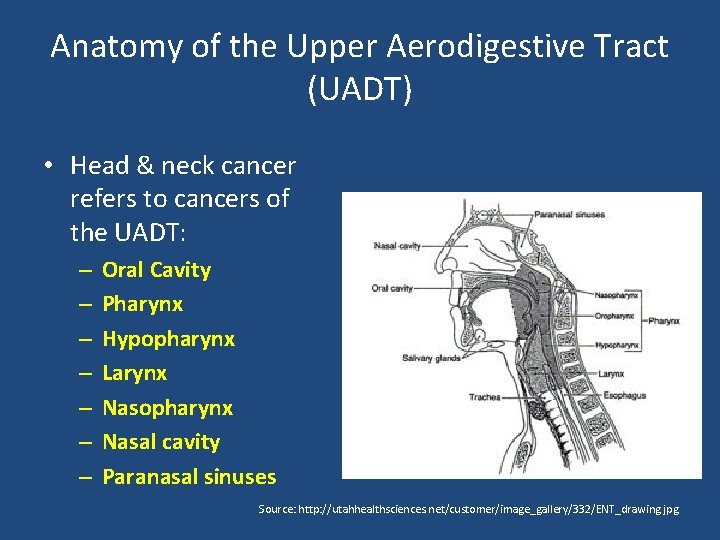
Anatomy of the Upper Aerodigestive Tract (UADT) • Head & neck cancer refers to cancers of the UADT: – – – – Oral Cavity Pharynx Hypopharynx Larynx Nasopharynx Nasal cavity Paranasal sinuses Source: http: //utahhealthsciences. net/customer/image_gallery/332/ENT_drawing. jpg

Epidemiology • Head and neck cancer in the US, 2008: – 47, 560 new cases (3. 3% of US cancers) – 9 th most common cancer – 11, 260 deaths (2% of US cancer deaths) – 14 th most common cause of cancer death • Globally, head and neck cancer results in: – 563, 826 new cases (5. 2% of world cancers) – 301, 408 deaths (4. 5% of world cancer deaths

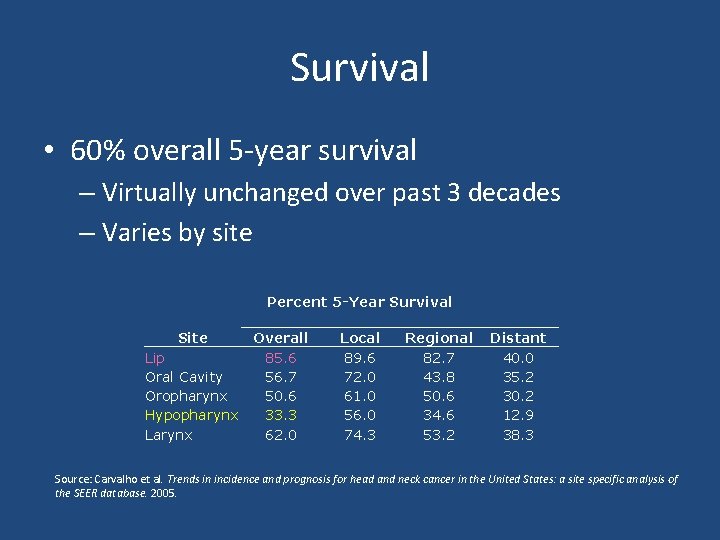
Survival • 60% overall 5 -year survival – Virtually unchanged over past 3 decades – Varies by site Site Lip Oral Cavity Oropharynx Hypopharynx Larynx Percent 5 -Year Survival Overall Local Regional Distant 85. 6 89. 6 82. 7 40. 0 56. 7 72. 0 43. 8 35. 2 50. 6 61. 0 50. 6 30. 2 33. 3 56. 0 34. 6 12. 9 62. 0 74. 3 53. 2 38. 3 Source: Carvalho et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site specific analysis of the SEER database. 2005.

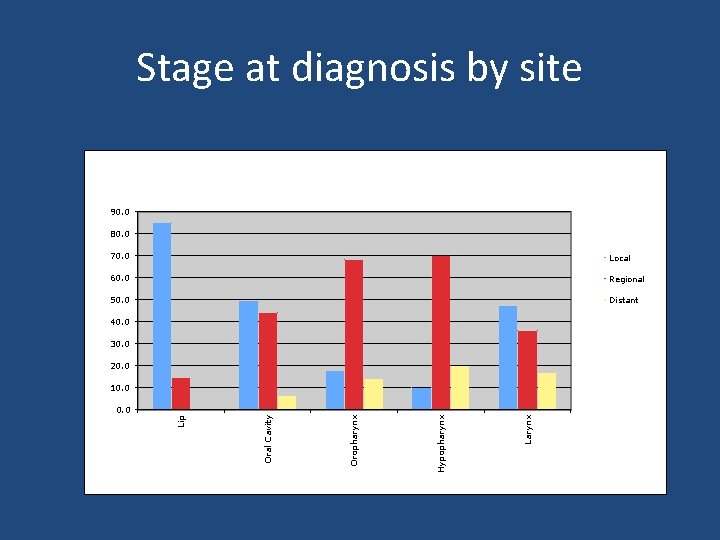
Stage at diagnosis by site 90. 0 80. 0 70. 0 Local 60. 0 Regional 50. 0 Distant 40. 0 30. 0 20. 0 10. 0 Larynx Hypopharynx Oral Cavity Lip 0. 0

Common Types of Head & Neck Cancer • Head and neck cancer is a heterogeneous category of malignancies • 90 -93% are head and neck squamous cell carcinomas (SCCHN) • 3 -6% are nasopharyngeal carcinomas (NPC) • 1 -3% are salivary cancers

Squamous Cell Carcinoma of the Head and Neck (SCCHN)

Clinical Presentation & Treatment • Currently no proven screening method except visual examination • Symptoms: hoarseness, dysphagia, odynophagia, ulcerations, bleeding • 1 st line treatment modalities: – Early stage: surgery (preferred) or radiation – Advanced stage: typically surgery + chemoradiation • Currently Cisplatin is treatment of choice, alone or in combination • EGFR inhibitors are in trials (cetuximab)

Epidemiology • 93% of head and neck cancers are SCCHN • Median age at diagnosis = 62 years – Although, incidence in adults < 45 increasing • Particularly for base of tongue and tonsillar SCC • Men are 2. 78 times as likely to develop SCCHN – 2. 59 times as likely to die from it • Higher incidence in African-Americans – Poorer 5 -year survival (16 -20% lower)

Prognostic Indicators • TNM stage (higher = worse) • Cervical nodal involvement is one of the strongest predictors (50% reduction in survival) – 2/3 SCCHN patients present with cervical mets – 10% present with distant mets – Extracapsular spread (ECS) further reduces survival • Other predictors – – – Nodal burden Perineural invasion Histologic grade Extent of necrosis Positive tumor margins

Recurrence and Second Primaries • > 50% of patients with locally advanced SCCHN experience recurrence within 2 years • 2 nd primaries occur in 15% of SCCHN patients at a rate of 3 -5% per year • Tumor recurrence or development of a second primary is a major reason for treatment failure and adversely impacts long-term survival

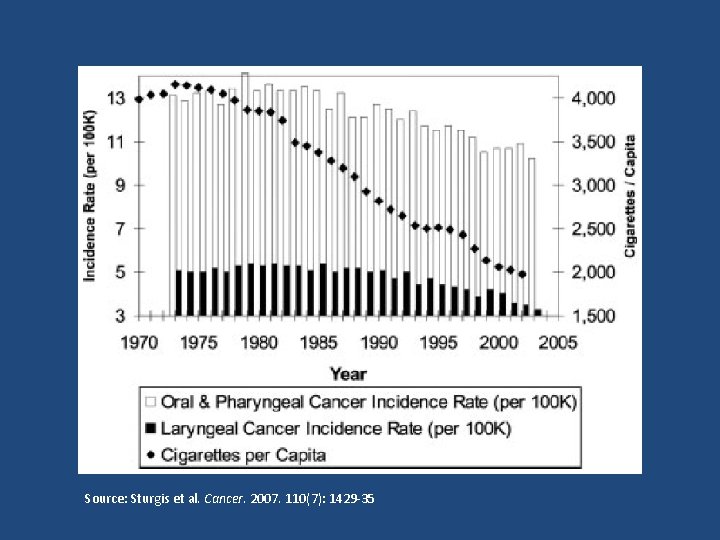
Tobacco • IARC classifies cigarette smoking as a Group 1 carcinogen and a causal factor for SCCHN • Linear dose-response for smoking & SCCHN – Duration > intensity, although both matter – 3 - to 10 -fold increase in risk for smokers vs. nonsmokers – 5 - to 25 -fold increase in risk for heavy smokers vs nonsmokers • Other forms of tobacco are also associated with SCCHN (pipe, cigar, chew) • Tobacco use also associated with 2 nd primary SCCHN

Source: Sturgis et al. Cancer. 2007. 110(7): 1429 -35

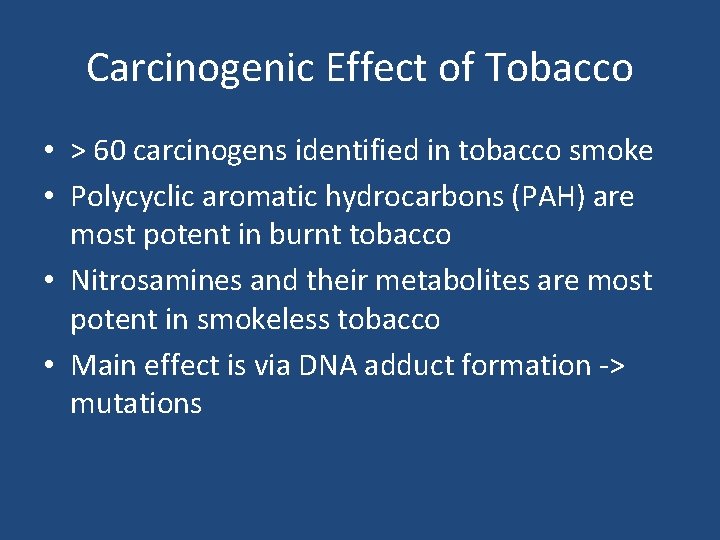
Carcinogenic Effect of Tobacco • > 60 carcinogens identified in tobacco smoke • Polycyclic aromatic hydrocarbons (PAH) are most potent in burnt tobacco • Nitrosamines and their metabolites are most potent in smokeless tobacco • Main effect is via DNA adduct formation -> mutations

Alcohol (Et. OH) • IARC classifies consumption of alcoholic beverages as causal for SCCHN • Contributive factor in ~75% of SCCHN • Dose-response effect – 50 g Et. OH/day is associated w/ 2 - to 3 -fold increased risk relative to non-drinkers – Heavy drinkers (> 100 g/day) = 4 - to 6. 5 -fold increase compared w/ non-drinkers • Also associated w/ 2 nd primary SCCHN

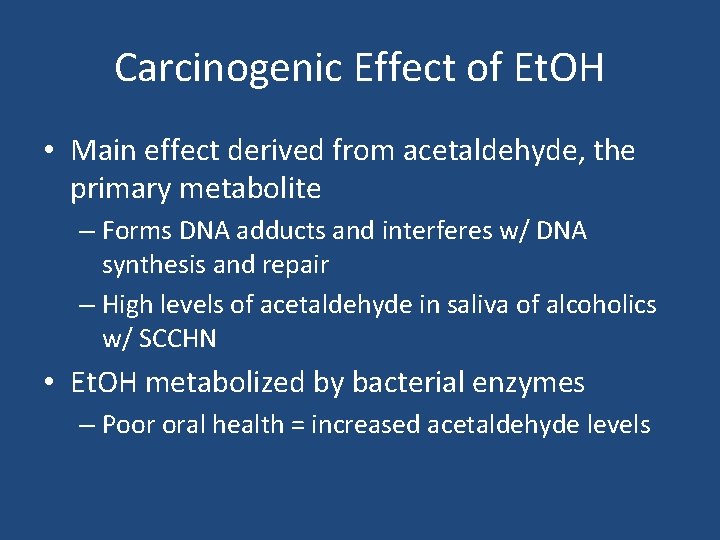
Carcinogenic Effect of Et. OH • Main effect derived from acetaldehyde, the primary metabolite – Forms DNA adducts and interferes w/ DNA synthesis and repair – High levels of acetaldehyde in saliva of alcoholics w/ SCCHN • Et. OH metabolized by bacterial enzymes – Poor oral health = increased acetaldehyde levels

Cigarette & Et. OH Synergy • There is an interaction between Et. OH and smoking, as it relates to SCCHN – The combined effect is multiplicative of the individual effects alone • Combined use accounts for 73% of SCCHN • Et. OH acts as a solvent for tobacco carcinogens • Smoking results in a shift in oral flora leading to increased acetaldehyde concentrations

Human Papillomavirus (HPV) • 25% of SCCHN associated w/ HPV – 45 -60% of oropharyngeal tumors, especially palatine and lingual tonsils • In contrast, 10% HPV+ in normal OP mucosa • 90 -95% of HPV+ tumors are HPV 16+ • HPV positive tumors represent a distinct clinical subset of tumors – Patients are younger, less likely to smoke/drink – Tumors are more likely basaloid and poorly diff – Better prognosis

HPV Oncogenic Effect • Primarily stems from action of E 6 & E 7 viral oncoproteins – E 6 binds and inactivates p 53 tumor suppressor • Loss of p 53 mediated apoptosis – E 7 binds and inactivates p. Rb tumor suppressor • Loss of G 1 -S phase checkpoint • p 16 overexpression is a surrogate marker for HPV-mediated SCCHN


Other Risk Factors • Environmental/Occupational – Indoor air pollution • Wood smoke or heating/cooking with fossil fuels – Chronic second-hand tobacco smoke exposure • 1. 5 - to 5 -fold increase in risk – Metal working – Exposure to toxins during mustard gas production • Diet – High in animal fat, low in fruits and vegetables associated w/ increased risk of SCCHN – Low folate intake may also be associated • Gastroesophageal reflux

Field Cancerization • The epithelium is chronically exposed to environmental carcinogens – Particularly true of smokers/drinkers • Mutations that confer a growth/survival advantage are clonally selected – Gradually replace normal epithelium – New mutations occur within these fields giving rise to subclones; eventually can become malignant – Can give rise to distinct but clonally related tumors – Evidenced by mutations/alterations in “negative” margins • This helps explain high recurrence/second primary rate of SCCHN

Nasopharyngeal Carcinoma (NPC)

Nasopharyngeal Carcinoma (NPC) • Arise from the epithelial lining of the nasal cavity/nasopharynx • Results from the interplay of environmental, genetic and viral risk factors • 70 -75% present with ear & nasal symptoms • 75% present with painless, enlarged cervical nodes

Treatment • Radiation or chemoradiation is typically the 1 st line of therapy – NPC is radiosensitive – Difficult to operate on nasopharynx • thus surgery is not generally used as 1 st line therapy for primary tumor – Surgery is used to remove cervical nodes with metastatic disease

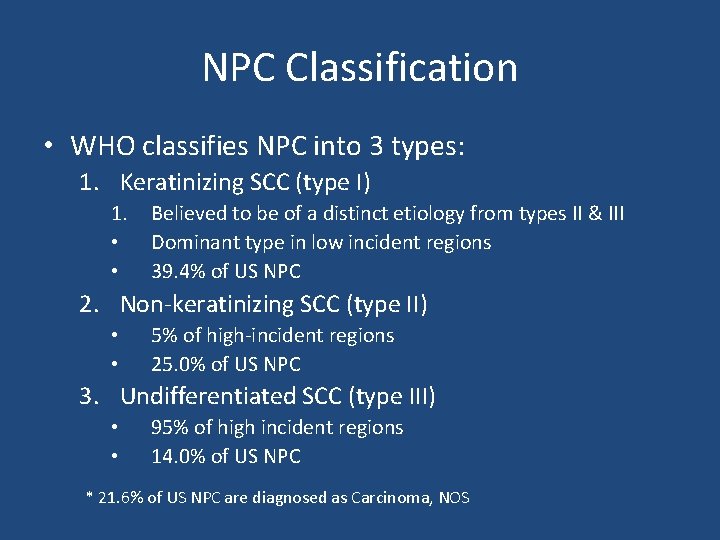
NPC Classification • WHO classifies NPC into 3 types: 1. Keratinizing SCC (type I) 1. • • Believed to be of a distinct etiology from types II & III Dominant type in low incident regions 39. 4% of US NPC 2. Non-keratinizing SCC (type II) • • 5% of high-incident regions 25. 0% of US NPC 3. Undifferentiated SCC (type III) • • 95% of high incident regions 14. 0% of US NPC * 21. 6% of US NPC are diagnosed as Carcinoma, NOS

Incidence & Mortality • 80, 000 cases with 50, 000 deaths annually – < 1 per 100, 000/year incidence – 23 rd most common cancer in the world • 2 -3 fold higher risk for males: females • Broad racial/ethnic and geographic variation – 4 th most common cancer in Hong Kong • Highest incidence in Asian, N African/Mid east, and Arctic populations



Migrant Studies • After migration, risk remains high – Chinese in US have 10 -20 fold increased risk compared to US Whites and Blacks – However, Chinese immigrants have ½ the risk of their counterparts in China – Conversely, Whites born in China or Philippines have an increased risk for NPC compared with US • Suggests both environmental and genetic components

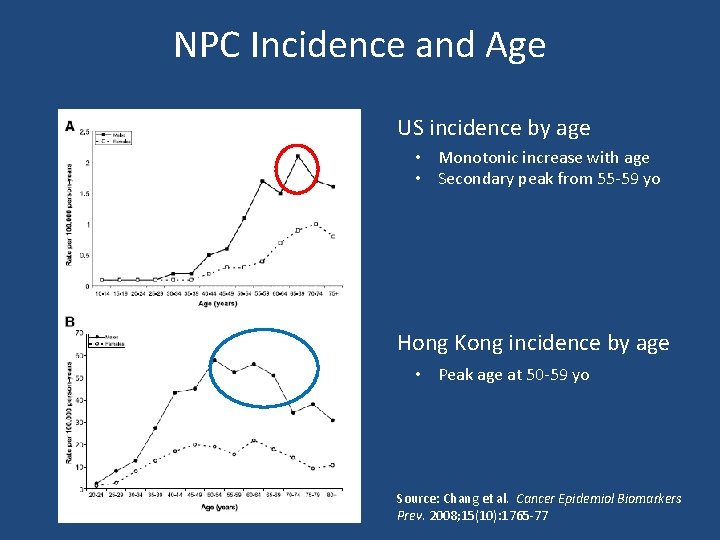
NPC Incidence and Age US incidence by age • Monotonic increase with age • Secondary peak from 55 -59 yo Hong Kong incidence by age • Peak age at 50 -59 yo Source: Chang et al. Cancer Epidemiol Biomarkers Prev. 2008; 15(10): 1765 -77

NPC Prognosis • Overall 5 -year survival of 65% (US) – Local: 80 -90% – Regional: 50 -70% – Advanced: poor • Varies by type: – Undifferentiated (type III) has best prognosis due to high radiosensitivity – Keratinizing (type I) have worst prognosis • more radioresistant

Epstein-Barr Virus (EBV) & NPC • Member of the herpesvirus family • Transmitted chiefly via saliva • Primarily targets B lymphocytes but also infects oropharyngeal tissue • EBV DNA detected in ~100% of type II and III NPC – Type I is not as consistent – Also detected in NP dysplasia

EBV Prevalence • > 90% of the world population is EBV seropositive (mostly latent infection) • Geographic variability in when infection occurs – Hong Kong: • 80% of kids infected by age 6 • ~100% by age 10 – US: • 50% of kids infected by age 5 • 95% of adults infected by age 40 • Tends to occur earlier in developing countries – Likely due to crowding and less hygienic conditions • 30 -50% adults develop mononucleosis after primary infection – Usually latent in children

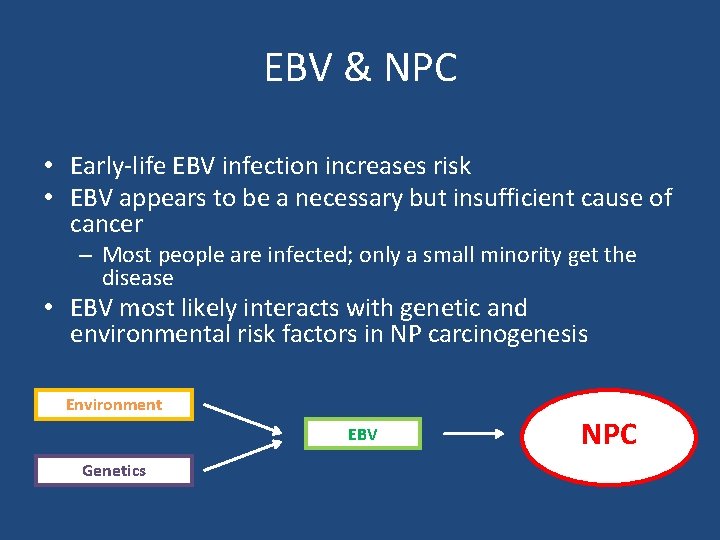
EBV & NPC • Early-life EBV infection increases risk • EBV appears to be a necessary but insufficient cause of cancer – Most people are infected; only a small minority get the disease • EBV most likely interacts with genetic and environmental risk factors in NP carcinogenesis Environment EBV Genetics NPC

Salt-Preserved Fish & Meat • Strongest non-viral risk factor (especially fish) • Associated with type II & III – Weekly consumption: 1. 4 -3. 2 fold increased risk – Daily consumption: 1. 8 -7. 5 fold increased risk • Childhood exposure increases risk > adult – Traditional weaning food in China • Salt is an ineffective preservative – Partial putrification – Contains: • • Nitrosamines (also found in tobacco) Bacterial mutagens Genotoxins EBV reactivating compounds

Smoking • 2 -6 fold increased risk of NPC – Particularly true for Type I – 2/3 of Type I attributable to smoking • IARC classifies smoking as a Group I carcinogen – Sufficient causal evidence for NPC

Occupational Exposures • Nasal cavity is the primary initial site of fume exposure • Difficult to study due to (relatively) rare exposures • Occupational exposures associated with NPC: – Formalin • 2 -4 fold increase in risk • Although not consistent across all studies • Supported by evidence from animal studies – Heat/combustion exposures – Wood dust – Chlorophenols

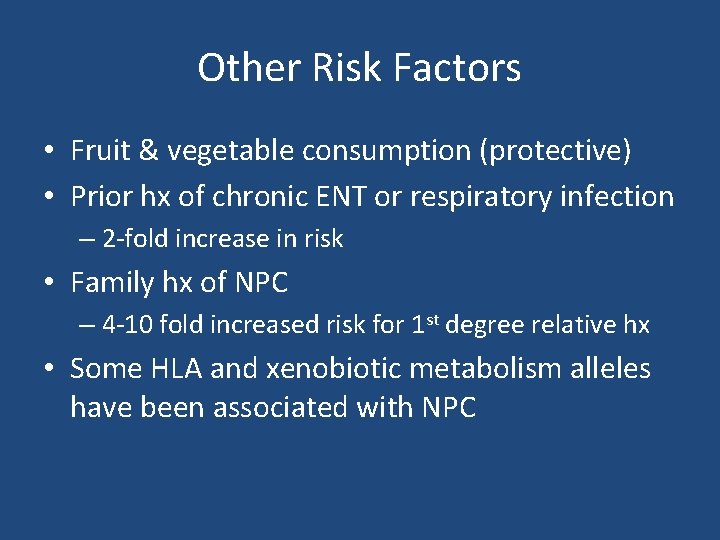
Other Risk Factors • Fruit & vegetable consumption (protective) • Prior hx of chronic ENT or respiratory infection – 2 -fold increase in risk • Family hx of NPC – 4 -10 fold increased risk for 1 st degree relative hx • Some HLA and xenobiotic metabolism alleles have been associated with NPC

Salivary Gland Cancers

Salivary Gland Cancers • 1 -3% of head and neck cancers • Incidence: 2 per 100, 000 people/yr – Incidence is rising in US • Mean age is 64 yo – 2/3 occur in people > 55, although occur at all ages • Heterogeneous group of malignancies – WHO lists 18 histologies

Site of Origin • Major salivary glands: 95% of cancers – 80% occur in the parotid • 20% of parotid tumors are malignant – 10% occur in the submandibular gland • > 50% are of submandibular tumors are malignant – 5% occur in the sublingual glands • > 50% of sublingual tumors are malignant • Minor salivary glands – Cancers mostly occur in the oral cavity, nasopharynx or nasal cavity

Clinical Presentation & Treatment • Present as fixed, painless lumps or swelling, with trismus, or facial nerve weakness – May present with or without lymphadenopathy • Surgery is the primary treatment modality – May be combined with radiation • Stage and grade correlate well with prognosis • After primary treatment, 50% experience local, regional, or distant mets

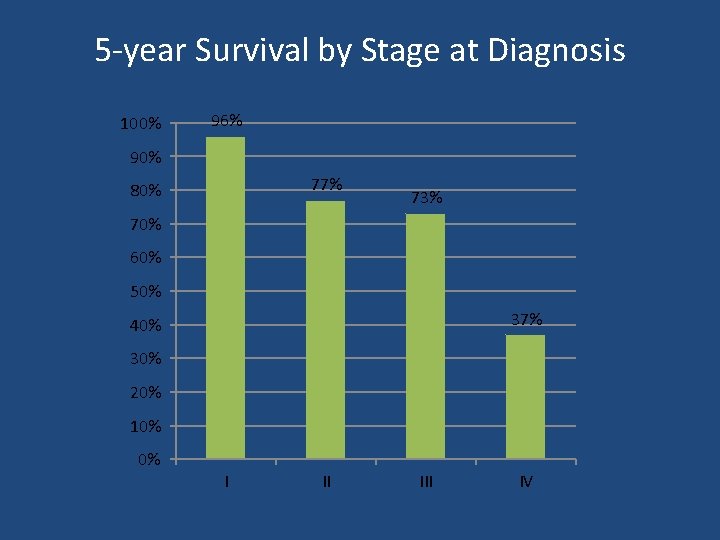
5 -year Survival by Stage at Diagnosis 100% 96% 90% 77% 80% 73% 70% 60% 50% 37% 40% 30% 20% 10% 0% I II IV

Salivary Gland Cancers • Major risk factors: – Radiation – Mixed results for occupational exposures • Because it is relatively rare, there are few epidemiologic studies available with adequate statistical power to identify associations

Mucoepidermoid Carcinoma (MEC) • • Most common: 30 -35% of salivary tumors Usually involves major salivary glands 3: 2 female: male ratio Mean age of diagnosis is in 5 th decade – Occurs at all ages – Most common salivary malignancy in children

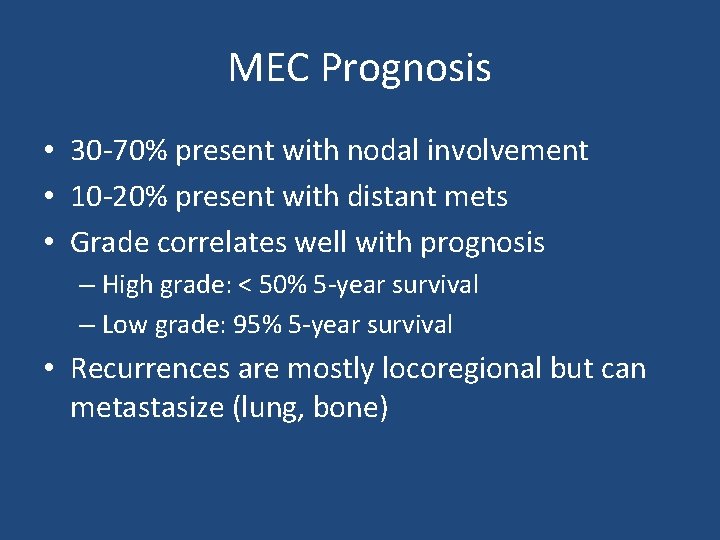
MEC Prognosis • 30 -70% present with nodal involvement • 10 -20% present with distant mets • Grade correlates well with prognosis – High grade: < 50% 5 -year survival – Low grade: 95% 5 -year survival • Recurrences are mostly locoregional but can metastasize (lung, bone)

Adenoid Cystic Carcinoma (ACC) • 2 nd most common salivary cancer • Occurs in both major and minor glands – Most common minor salivary malignancy • 3: 2 female: male ratio • 4 th – 6 th decade is the peak occurance – But occurs at all ages

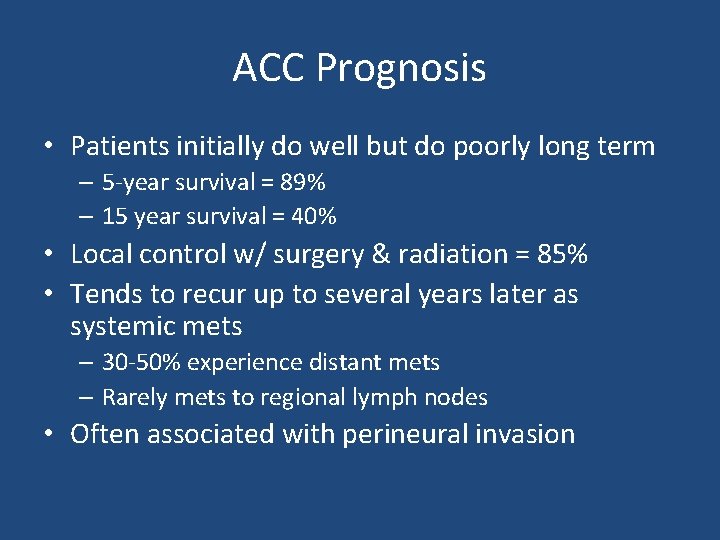
ACC Prognosis • Patients initially do well but do poorly long term – 5 -year survival = 89% – 15 year survival = 40% • Local control w/ surgery & radiation = 85% • Tends to recur up to several years later as systemic mets – 30 -50% experience distant mets – Rarely mets to regional lymph nodes • Often associated with perineural invasion

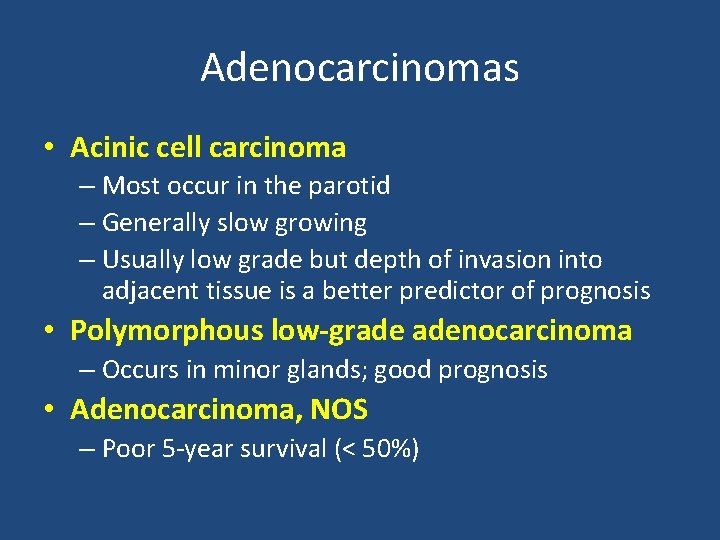
Adenocarcinomas • Acinic cell carcinoma – Most occur in the parotid – Generally slow growing – Usually low grade but depth of invasion into adjacent tissue is a better predictor of prognosis • Polymorphous low-grade adenocarcinoma – Occurs in minor glands; good prognosis • Adenocarcinoma, NOS – Poor 5 -year survival (< 50%)

Rare Adenocarcinomas • Typically low grade (good prognosis): – Basal cell adenocarcinoma – Clear cell adenocarcinoma – Cystadenocarcinoma – Sebaceous adenocarcinoma – Mucinous adenocarcinoma • Typically high grade (poor prognosis) – Oncocytic adenocarcinoma – Salivary duct carcinoma

Malignant Mixed Tumors • Tend to occur in the major glands • Prognosis depends upon grade 1. Carcinoma ex pleomorphic adenoma – Account for the majority of MMTs 2. Carcinosarcomas 3. Metastasizing mixed tumor very, very rare

Other Salivary Malignancies • Squamous cell carcinoma • Epithelial-myoepithelial carcinoma • Anaplastic small cell – Minor glands; quick growing • Undifferentiated carcinoma (poor outcome) – Large cell undifferentiated – Lymphoepithelial carcinoma • Higher incidence in Inuits

Questions
 Risk factors of head and neck cancer
Risk factors of head and neck cancer My ukulele has a body a neck and a head song
My ukulele has a body a neck and a head song Tnm 8 head and neck
Tnm 8 head and neck This is home ukulele
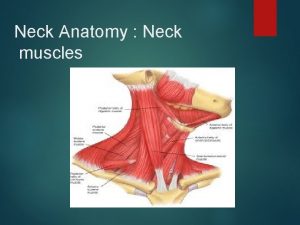
This is home ukulele Muscular system assignment
Muscular system assignment Head and neck muscles
Head and neck muscles Regional write up head face and neck
Regional write up head face and neck Eccomyosis
Eccomyosis What has a neck but no head
What has a neck but no head Simple indexing
Simple indexing Positive suction head and negative suction head
Positive suction head and negative suction head Thesourceagents
Thesourceagents Advantages and disadvantages of nutritional epidemiology
Advantages and disadvantages of nutritional epidemiology Descriptive vs analytical epidemiology
Descriptive vs analytical epidemiology Descriptive vs analytical epidemiology
Descriptive vs analytical epidemiology Certification board of infection control and epidemiology
Certification board of infection control and epidemiology Person place time epidemiology
Person place time epidemiology The attacking firm goes head-to-head with its competitor.
The attacking firm goes head-to-head with its competitor. Informtika
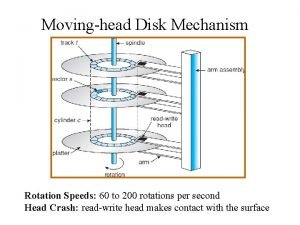
Informtika The head of a moving head disk
The head of a moving head disk Parts of the head
Parts of the head What is a tonic syllable
What is a tonic syllable Biceps femoris short head
Biceps femoris short head Pre-head head tonic syllable tail
Pre-head head tonic syllable tail The head of moving head disk with 100 tracks
The head of moving head disk with 100 tracks Contoh relative risk
Contoh relative risk Logistic regression epidemiology
Logistic regression epidemiology How to calculate prevalence
How to calculate prevalence Cross sectional study advantages and disadvantages
Cross sectional study advantages and disadvantages Attack rate formula
Attack rate formula Bibliography of epidemiology
Bibliography of epidemiology Recall bias example
Recall bias example Attack rate calculation
Attack rate calculation Gate frame epidemiology
Gate frame epidemiology Biological plausibility
Biological plausibility Defination of proportion
Defination of proportion Defination of epidemiology
Defination of epidemiology Cholera 1817
Cholera 1817 What is descriptive study in epidemiology
What is descriptive study in epidemiology Spurious association
Spurious association Field epidemiology ppt
Field epidemiology ppt Epidemiology
Epidemiology Gordon epidemiology
Gordon epidemiology Epidemiology kept simple
Epidemiology kept simple Diabetic ketoacidosis epidemiology
Diabetic ketoacidosis epidemiology Distribution in epidemiology
Distribution in epidemiology Effect modification vs confounding
Effect modification vs confounding Distribution in epidemiology
Distribution in epidemiology Ramboman
Ramboman Epidemiology description
Epidemiology description Prevalensi adalah
Prevalensi adalah How dr. wafaa elsadr epidemiology professor
How dr. wafaa elsadr epidemiology professor Nutritional epidemiology
Nutritional epidemiology Ookinite
Ookinite Attack rate epidemiology
Attack rate epidemiology