Test Review Properties of Matter Matter Matter is








- Slides: 18

Test Review Properties of Matter

Matter § Matter is a term used to describe anything that has mass and takes up space.

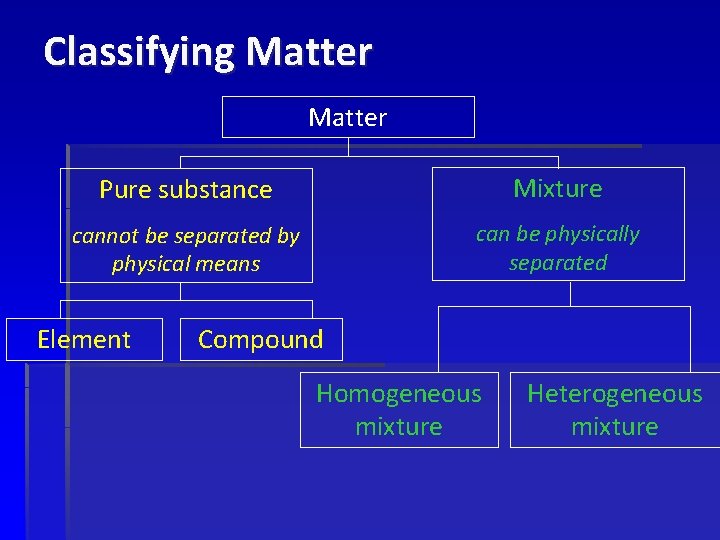
Classifying Matter Pure substance Mixture cannot be separated by physical means can be physically separated Element Compound Homogeneous mixture Heterogeneous mixture

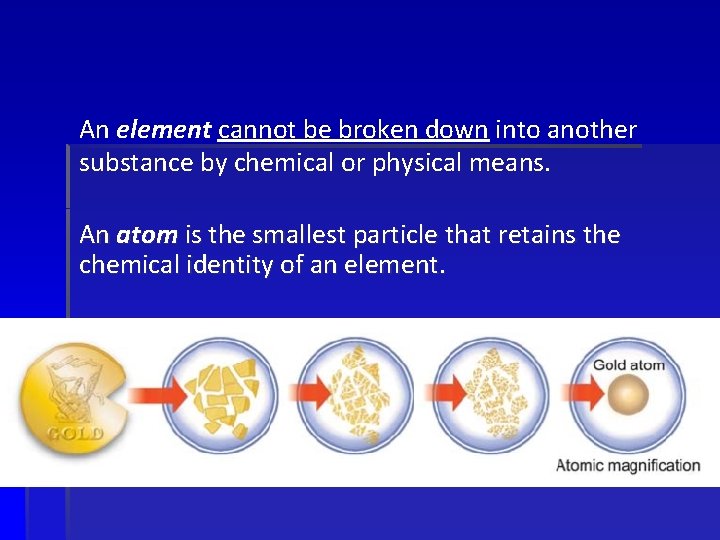
An element cannot be broken down into another substance by chemical or physical means. An atom is the smallest particle that retains the chemical identity of an element.

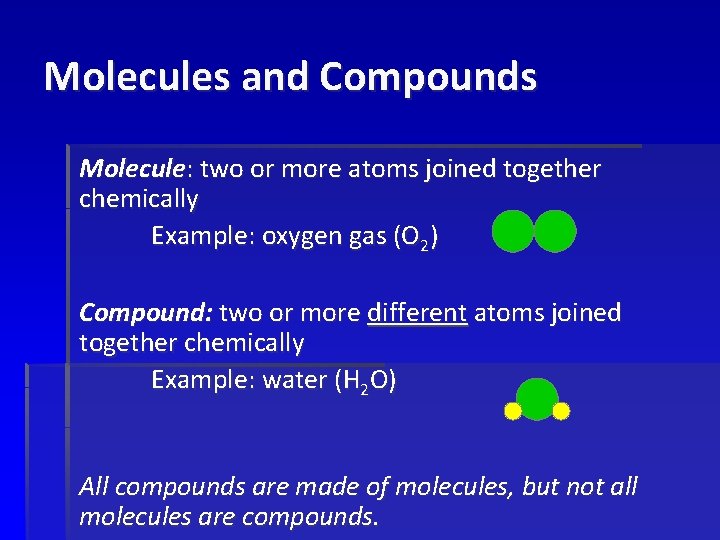
Molecules and Compounds Molecule: two or more atoms joined together chemically Example: oxygen gas (O 2) Compound: two or more different atoms joined together chemically Example: water (H 2 O) All compounds are made of molecules, but not all molecules are compounds.

Mixtures § Homogeneous mixture: A mixture that is the same throughout Cannot be easily separated ex) Chocolate milk, salt water § Heterogeneous mixture: A mixture that is not the same throughout Can be easily separated ex) Oil with water, trail mix, salad

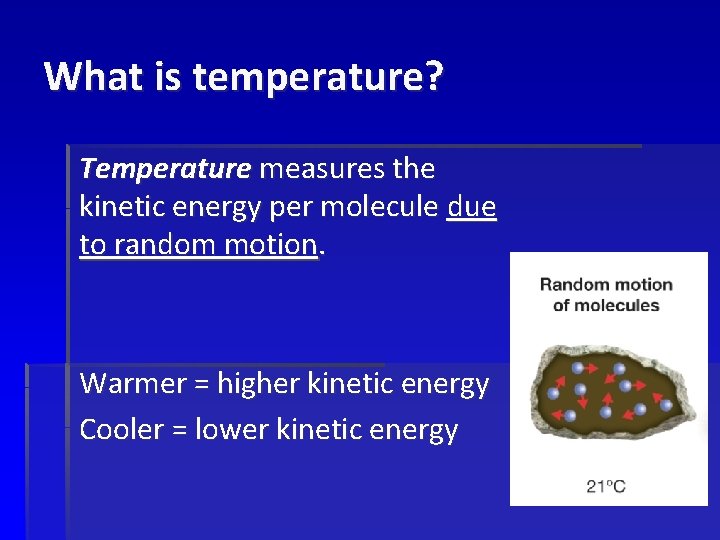
What is temperature? Temperature measures the kinetic energy per molecule due to random motion. Warmer = higher kinetic energy Cooler = lower kinetic energy

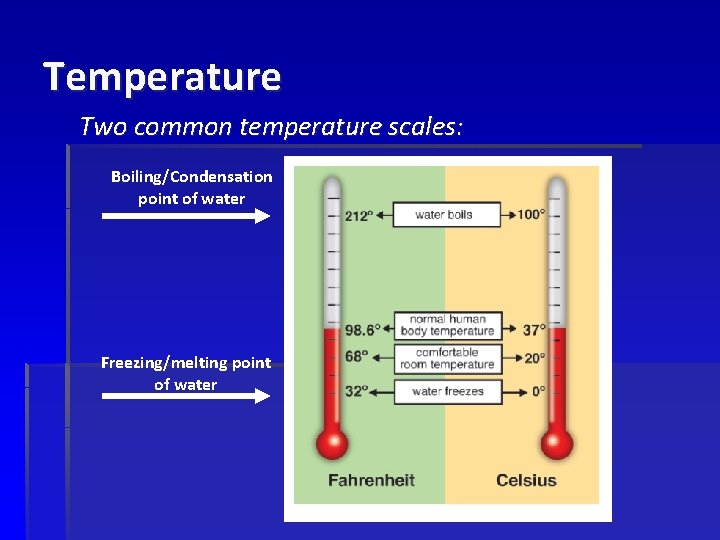
Temperature Two common temperature scales: Boiling/Condensation point of water Freezing/melting point of water

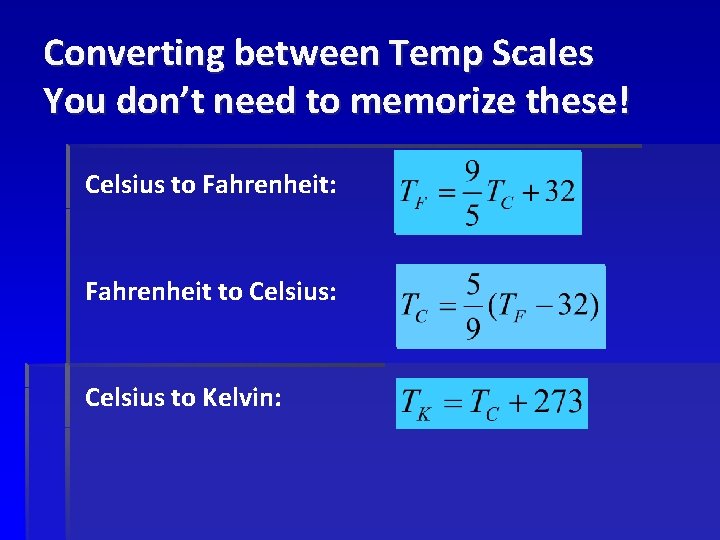
Converting between Temp Scales You don’t need to memorize these! Celsius to Fahrenheit: Fahrenheit to Celsius: Celsius to Kelvin:

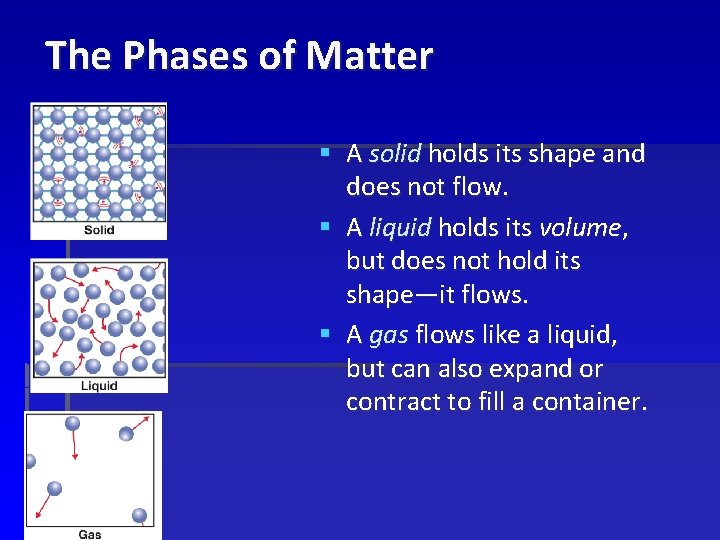
The Phases of Matter § A solid holds its shape and does not flow. § A liquid holds its volume, but does not hold its shape—it flows. § A gas flows like a liquid, but can also expand or contract to fill a container.

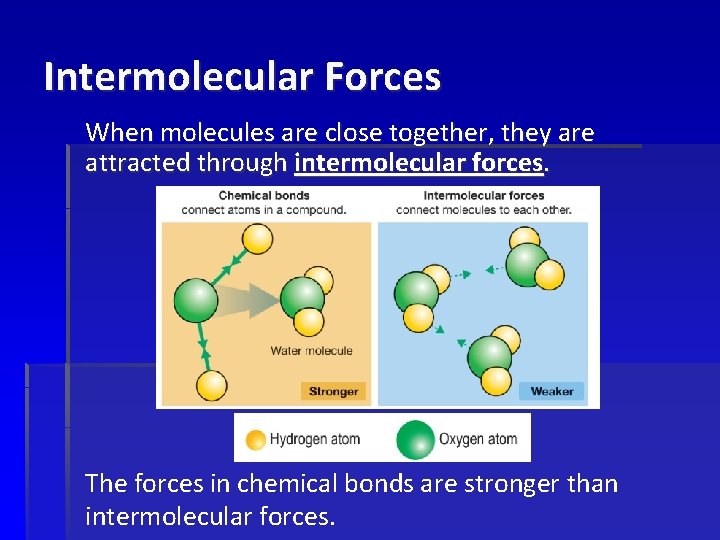
Intermolecular Forces When molecules are close together, they are attracted through intermolecular forces. The forces in chemical bonds are stronger than intermolecular forces.

Phase Changes Process Melting Freezing Condensing Boiling Sublimation Deposition Starting Ending Phase solid liquid solid gas liquid gas solid

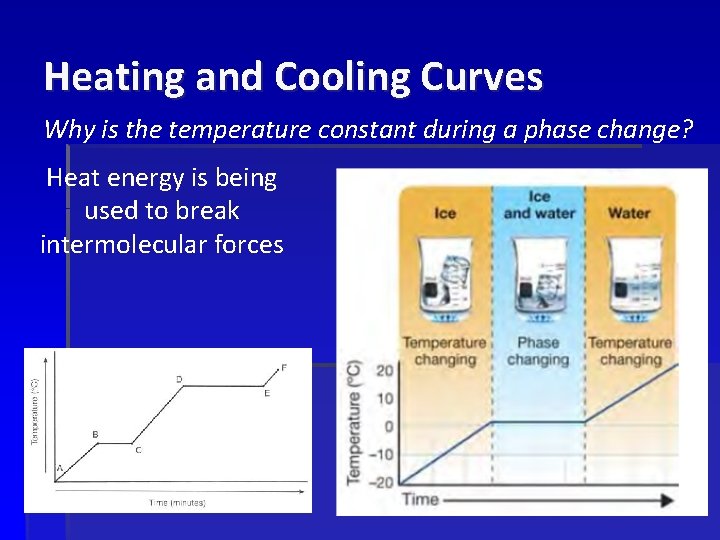
Heating and Cooling Curves Why is the temperature constant during a phase change? Heat energy is being used to break intermolecular forces Freezing

Chemical and Physical Changes Example Physical Breaking Candy X Boiling Water X Chemical Baking Soda/Vinegar X Burning Paper X Melting Ice X § Physical Changes – You end up with the same substance § Chemical Changes – End up with a new substance

Density: how much mass is in a given volume of material Mass: the amount of “stuff” (particles) Volume: How much space something takes up. All matter has volume! § Styrofoam ball vs. Steel ball Same volume, different mass!

Calculating Density (g/m. L or g/cm 3) Units of density: - liquids: g/m. L - solids: g/cm 3 Mass (g) Volume (m. L or cm 3)

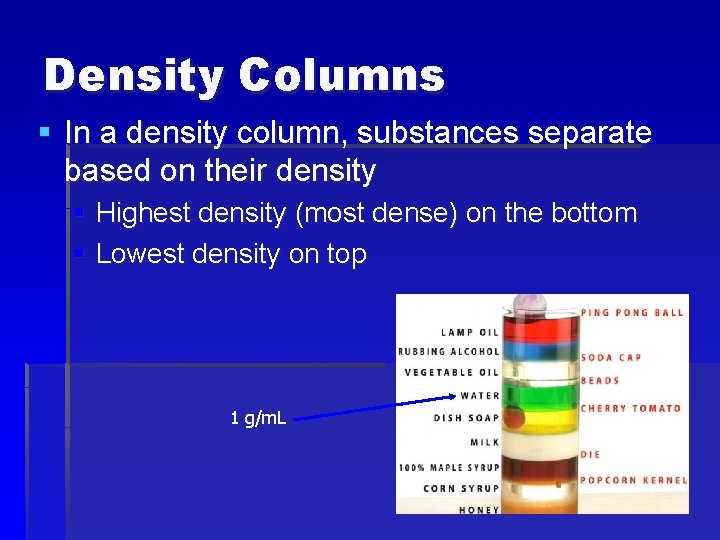
Density Columns § In a density column, substances separate based on their density § Highest density (most dense) on the bottom § Lowest density on top 1 g/m. L

Throwback Questions § § § Dimensional analysis Measurements Graphing and variables
 Algebra 2 unit 1 practice test
Algebra 2 unit 1 practice test Extensive examples
Extensive examples Chemical properties of citric acid
Chemical properties of citric acid Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Uncontrollable spending ap gov
Uncontrollable spending ap gov Narrative review vs systematic review
Narrative review vs systematic review Example of inclusion and exclusion criteria
Example of inclusion and exclusion criteria Narrative review vs systematic review
Narrative review vs systematic review Properties of liquid
Properties of liquid Properties of matter vocabulary
Properties of matter vocabulary Concept map properties of matter
Concept map properties of matter Objectives of properties of matter
Objectives of properties of matter General properties of matter
General properties of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Measurable properties examples
Measurable properties examples States of matter jeopardy
States of matter jeopardy Properties of matter jeopardy
Properties of matter jeopardy Volume properties of matter
Volume properties of matter


































