Targeting angiogenesis in Lung cancer Lucio Crin MD







- Slides: 37

Targeting angiogenesis in Lung cancer Lucio Crinò, MD Medical Oncology Department University Hospital Perugia, Italy

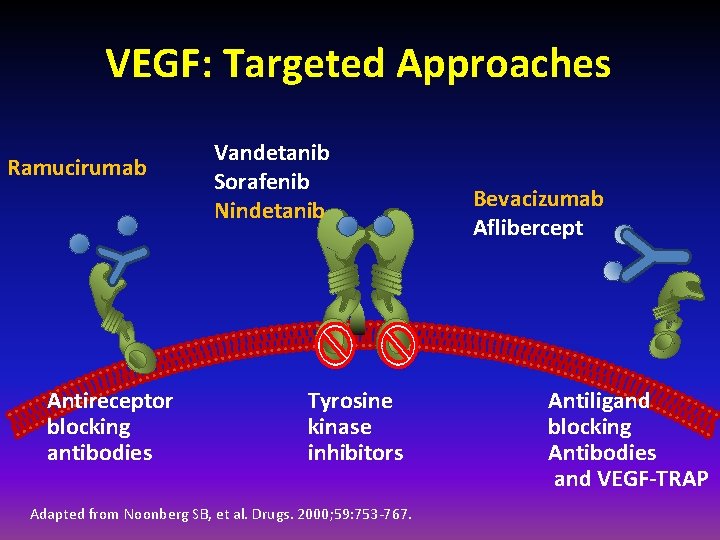
VEGF: Targeted Approaches Ramucirumab Antireceptor blocking antibodies Vandetanib Sorafenib Nindetanib Tyrosine kinase inhibitors Adapted from Noonberg SB, et al. Drugs. 2000; 59: 753 -767. Bevacizumab Aflibercept Antiligand blocking Antibodies and VEGF-TRAP

Outline • Antiangiogenic agents in the first-line setting • Bevacizumab • Recently completed randomised trials • Multi-targeted antiangiogenic orally administered TKIs • Vascular disrupting agents • Antiangiogenic agents in a relapsed/refractory setting • Recently completed randomised trials • Multi-targeted antiangiogenic orally administered TKIs -Nindetanib • Monoclonal antibodies and decoy receptors • Vascular disrupting agents • Other investigational agents • Ramucirumab

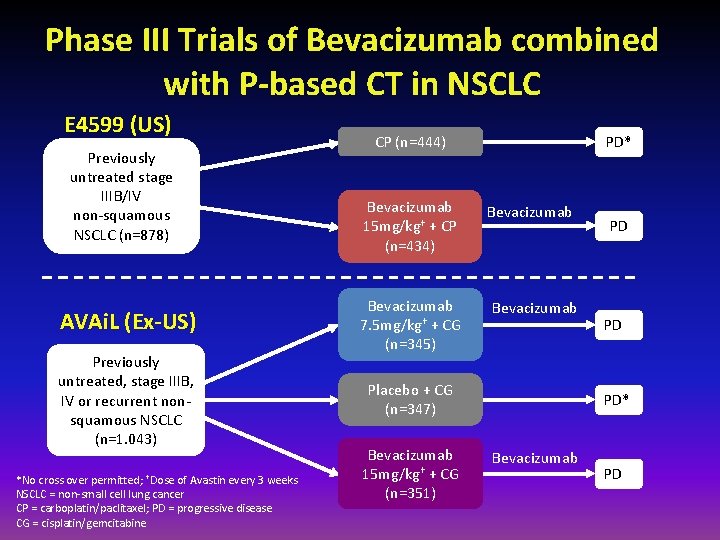
Phase III Trials of Bevacizumab combined with P-based CT in NSCLC E 4599 (US) Previously untreated stage IIIB/IV non-squamous NSCLC (n=878) AVAi. L (Ex-US) Previously untreated, stage IIIB, IV or recurrent nonsquamous NSCLC (n=1. 043) *No cross over permitted; †Dose of Avastin every 3 weeks NSCLC = non-small cell lung cancer CP = carboplatin/paclitaxel; PD = progressive disease CG = cisplatin/gemcitabine CP (n=444) Bevacizumab 15 mg/kg† + CP (n=434) Bevacizumab 7. 5 mg/kg† + CG (n=345) PD* Bevacizumab Placebo + CG (n=347) Bevacizumab 15 mg/kg† + CG (n=351) PD PD PD* Bevacizumab PD

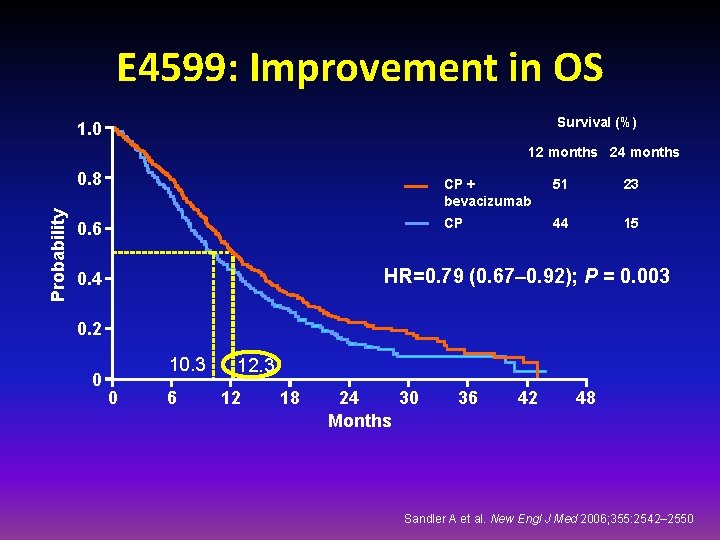
E 4599: Improvement in OS Survival (%) 1. 0 12 months 24 months Probability 0. 8 0. 6 CP + bevacizumab 51 23 CP 44 15 HR=0. 79 (0. 67– 0. 92); P = 0. 003 0. 4 0. 2 0 10. 3 0 6 12. 3 12 18 24 30 Months 36 42 48 Sandler A et al. New Engl J Med 2006; 355: 2542– 2550

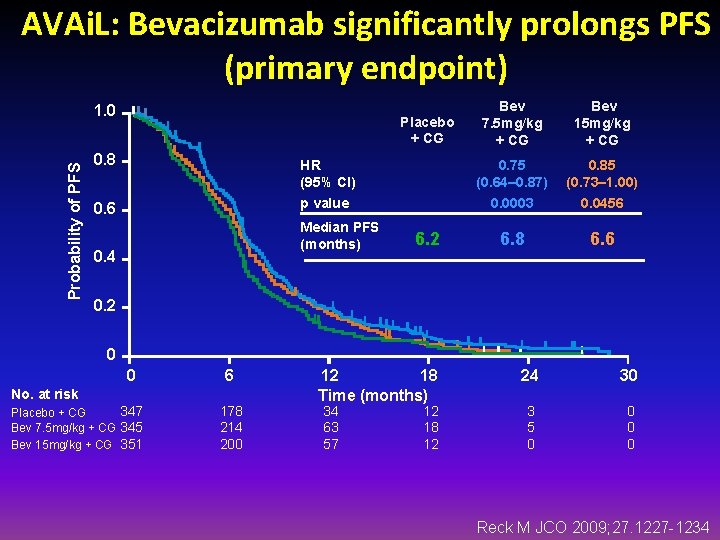
AVAi. L: Bevacizumab significantly prolongs PFS (primary endpoint) Probability of PFS 1. 0 Placebo + CG Bev 7. 5 mg/kg + CG Bev 15 mg/kg + CG 0. 8 HR (95% CI) 0. 75 (0. 64– 0. 87) 0. 85 (0. 73– 1. 00) 0. 6 p value 0. 0003 0. 0456 6. 8 6. 6 Median PFS (months) 0. 4 6. 2 0 0 6 No. at risk 347 Placebo + CG Bev 7. 5 mg/kg + CG 345 Bev 15 mg/kg + CG 351 178 214 200 12 18 Time (months) 34 63 57 12 18 12 24 30 3 5 0 0 Reck M JCO 2009; 27. 1227 -1234

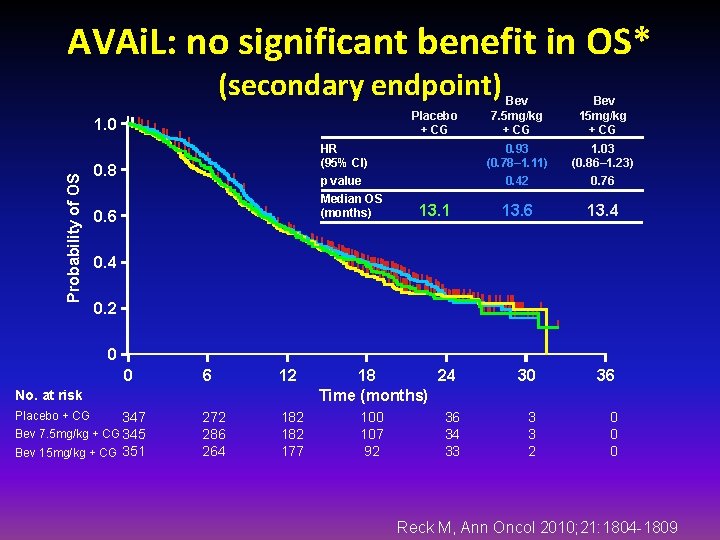
AVAi. L: no significant benefit in OS* (secondary endpoint) Placebo + CG Probability of OS 1. 0 HR (95% CI) p value 0. 8 Median OS (months) 0. 6 13. 1 Bev 7. 5 mg/kg + CG Bev 15 mg/kg + CG 0. 93 (0. 78– 1. 11) 0. 42 1. 03 (0. 86– 1. 23) 0. 76 13. 4 30 36 3 3 2 0 0. 4 0. 2 0 0 6 12 272 286 264 182 177 No. at risk Placebo + CG 347 Bev 7. 5 mg/kg + CG 345 Bev 15 mg/kg + CG 351 18 24 Time (months) 100 107 92 36 34 33 Reck M, Ann Oncol 2010; 21: 1804 -1809

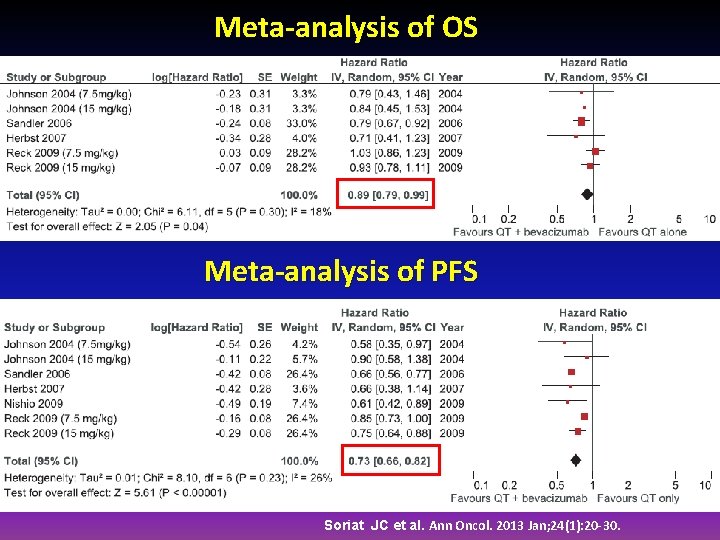
Meta-analysis of OS Meta-analysis of PFS Soriat JC et al. Ann Oncol. 2013 Jan; 24(1): 20 -30.

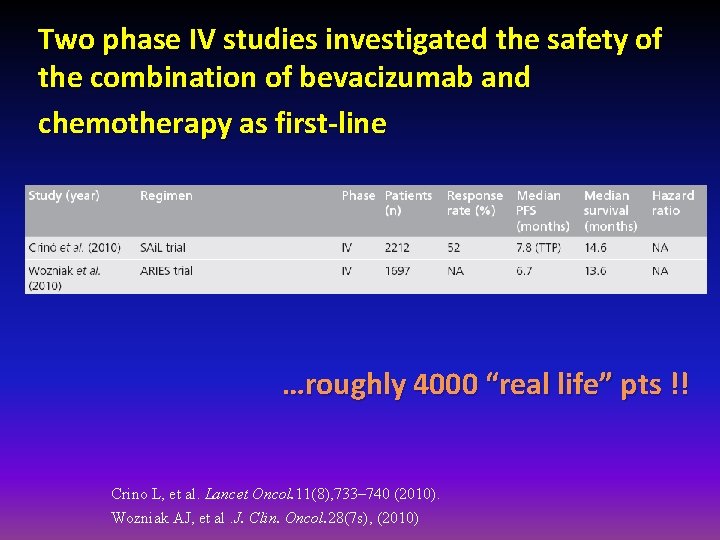
Two phase IV studies investigated the safety of the combination of bevacizumab and chemotherapy as first-line …roughly 4000 “real life” pts !! Crino L, et al. Lancet Oncol. 11(8), 733– 740 (2010). Wozniak AJ, et al. J. Clin. Oncol. 28(7 s), (2010)

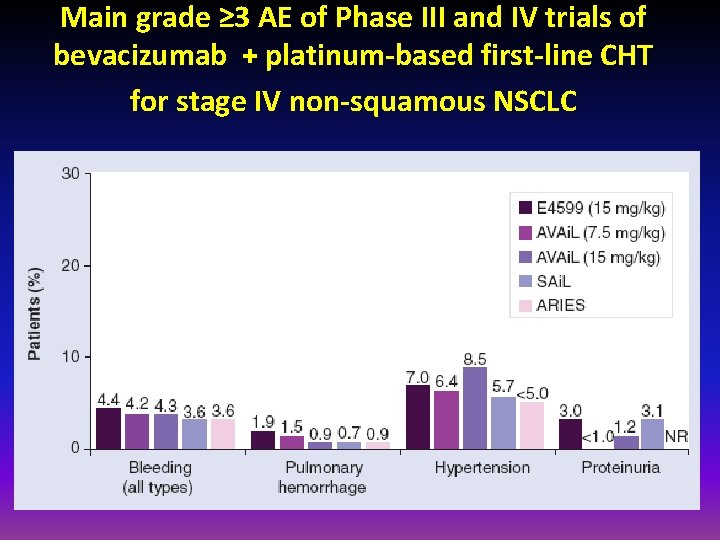
Main grade ≥ 3 AE of Phase III and IV trials of bevacizumab + platinum-based first-line CHT for stage IV non-squamous NSCLC

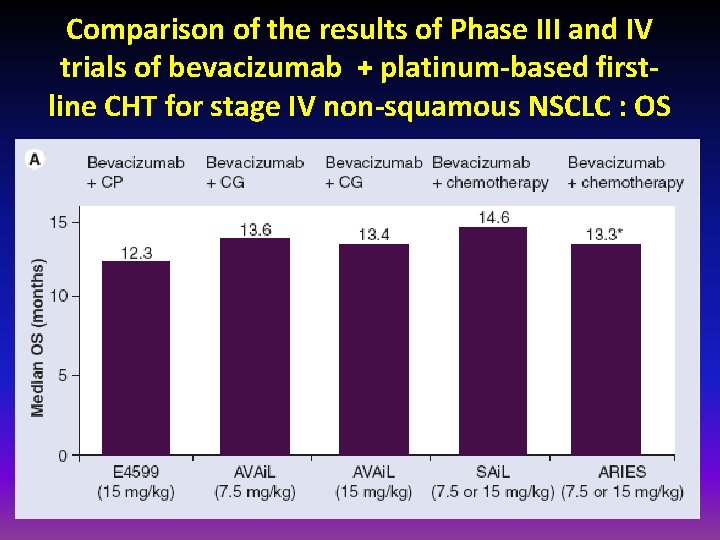
Comparison of the results of Phase III and IV trials of bevacizumab + platinum-based firstline CHT for stage IV non-squamous NSCLC : OS

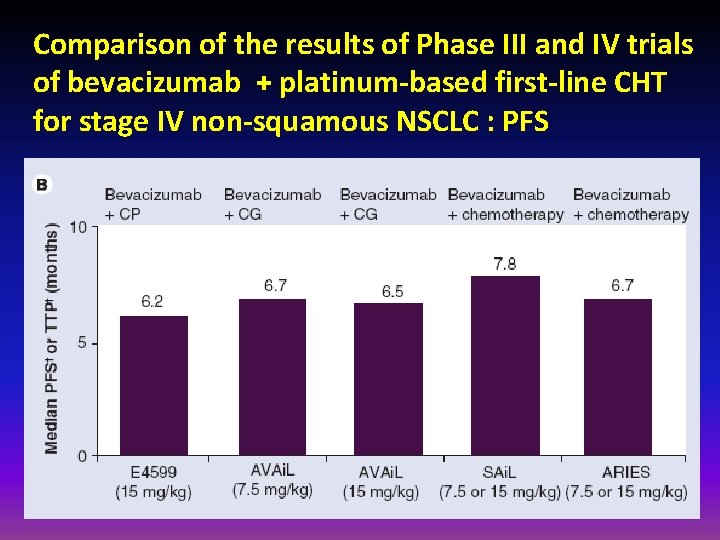
Comparison of the results of Phase III and IV trials of bevacizumab + platinum-based first-line CHT for stage IV non-squamous NSCLC : PFS

Outline • Antiangiogenic agents in the first-line setting • Bevacizumab • Recently completed randomised trials • Multi-targeted antiangiogenic orally administered TKIs • Vascular disrupting agents • Antiangiogenic agents in a relapsed/refractory setting • Recently completed randomised trials • Multi-targeted antiangiogenic orally administered TKIs -Nindetanib • Monoclonal antibodies and decoy receptors • Vascular disrupting agents • Other investigational agents • Ramucirumab

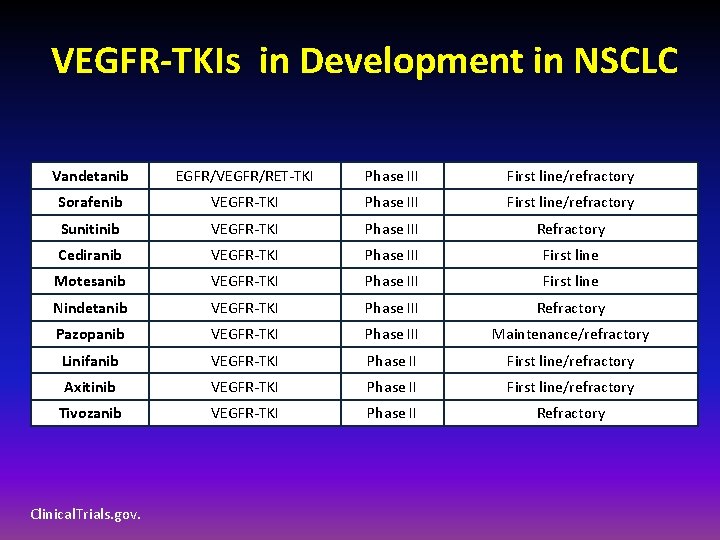
VEGFR-TKIs in Development in NSCLC Vandetanib EGFR/VEGFR/RET-TKI Phase III First line/refractory Sorafenib VEGFR-TKI Phase III First line/refractory Sunitinib VEGFR-TKI Phase III Refractory Cediranib VEGFR-TKI Phase III First line Motesanib VEGFR-TKI Phase III First line Nindetanib VEGFR-TKI Phase III Refractory Pazopanib VEGFR-TKI Phase III Maintenance/refractory Linifanib VEGFR-TKI Phase II First line/refractory Axitinib VEGFR-TKI Phase II First line/refractory Tivozanib VEGFR-TKI Phase II Refractory Clinical. Trials. gov.

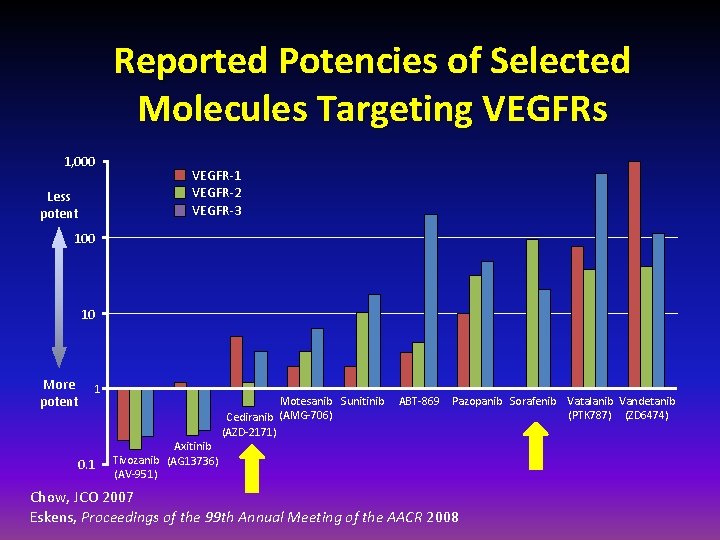
Reported Potencies of Selected Molecules Targeting VEGFRs 1, 000 Less potent VEGFR-1 VEGFR-2 VEGFR-3 100 10 More potent 1 0. 1 Axitinib Tivozanib (AG 13736) (AV-951) Motesanib Sunitinib (AMG-706) Cediranib (AZD-2171) ABT-869 Pazopanib Sorafenib Vatalanib Vandetanib (PTK 787) (ZD 6474) Chow, JCO 2007 Eskens, Proceedings of the 99 th Annual Meeting of the AACR 2008

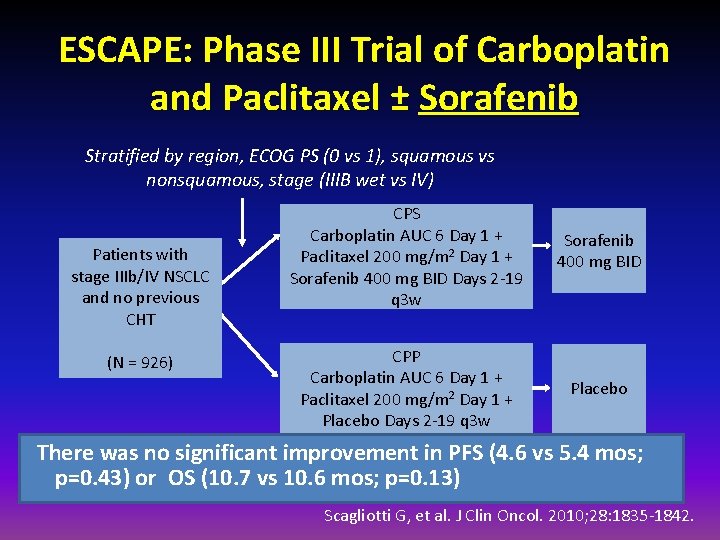
ESCAPE: Phase III Trial of Carboplatin and Paclitaxel ± Sorafenib Stratified by region, ECOG PS (0 vs 1), squamous vs nonsquamous, stage (IIIB wet vs IV) Patients with stage IIIb/IV NSCLC and no previous CHT (N = 926) CPS Carboplatin AUC 6 Day 1 + Paclitaxel 200 mg/m 2 Day 1 + Sorafenib 400 mg BID Days 2 -19 q 3 w Sorafenib 400 mg BID CPP Carboplatin AUC 6 Day 1 + Paclitaxel 200 mg/m 2 Day 1 + Placebo Days 2 -19 q 3 w Placebo There was no significant improvement in PFS (4. 6 vs 5. 4 mos; p=0. 43) or OS (10. 7 vs 10. 6 mos; p=0. 13) Scagliotti G, et al. J Clin Oncol. 2010; 28: 1835 -1842.

Outline • Antiangiogenic agents in the first-line setting • Bevacizumab • Recently completed randomised trials • Multi-targeted antiangiogenic orally administered TKIs • Vascular disrupting agents • Antiangiogenic agents in a relapsed/refractory setting • Recently completed randomised trials • Multi-targeted antiangiogenic orally administered TKIs -Nindetanib -Vandetanib • Monoclonal antibodies and decoy receptors • Vascular disrupting agents • Other investigational agents • Ramucirumab

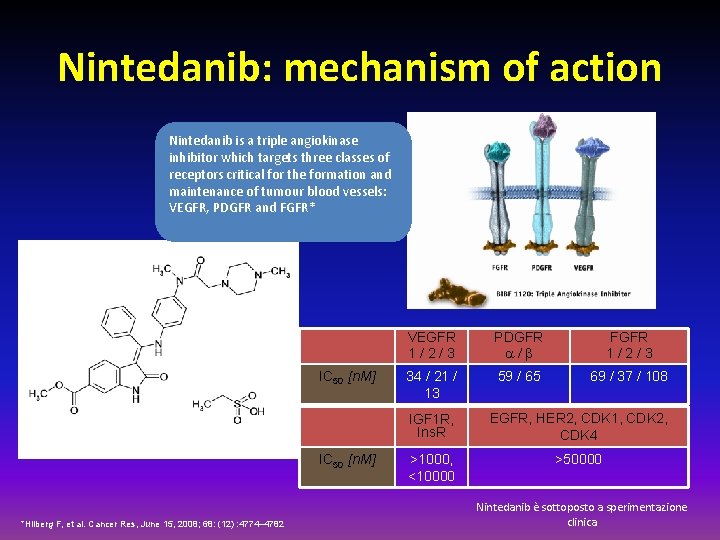
Nintedanib: mechanism of action Nintedanib is a triple angiokinase inhibitor which targets three classes of receptors critical for the formation and maintenance of tumour blood vessels: VEGFR, PDGFR and FGFR* IC 50 [n. M] *Hilberg F, et al. Cancer Res, June 15, 2008; 68: (12) : 4774– 4782 VEGFR 1/2/3 PDGFR a/b FGFR 1/2/3 34 / 21 / 13 59 / 65 69 / 37 / 108 IGF 1 R, Ins. R EGFR, HER 2, CDK 1, CDK 2, CDK 4 >1000, <10000 >50000 Nintedanib è sottoposto a sperimentazione clinica

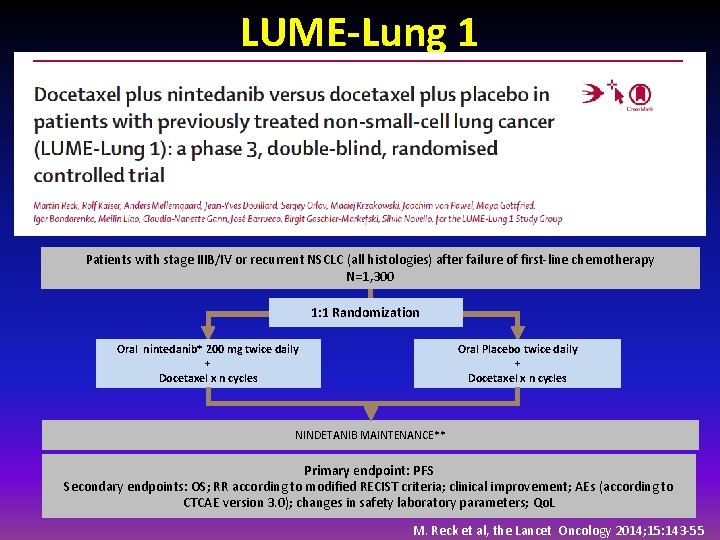
LUME-Lung 1 Patients with stage IIIB/IV or recurrent NSCLC (all histologies) after failure of first-line chemotherapy N=1, 300 1: 1 Randomization Oral nintedanib* 200 mg twice daily + Docetaxel x n cycles Oral Placebo twice daily + Docetaxel x n cycles NINDETANIB MAINTENANCE** Primary endpoint: PFS Secondary endpoints: OS; RR according to modified RECIST criteria; clinical improvement; AEs (according to CTCAE version 3. 0); changes in safety laboratory parameters; Qo. L M. Reck et al, the Lancet Oncology 2014; 15: 143 -55

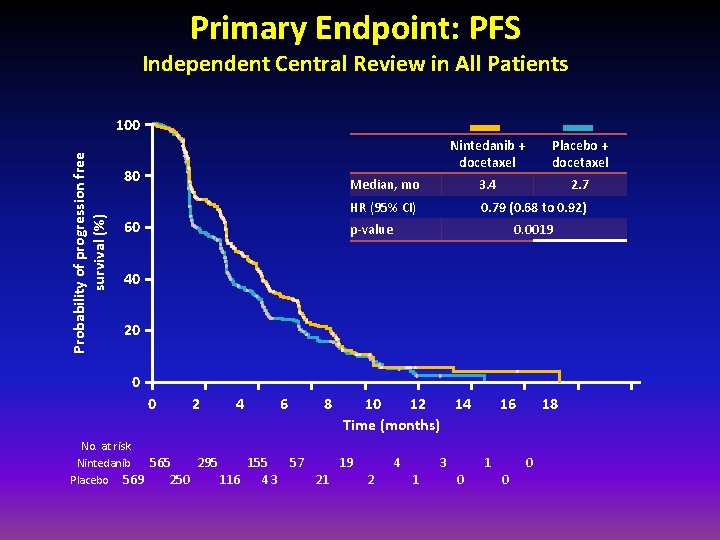
Primary Endpoint: PFS Independent Central Review in All Patients Probability of progression free survival (%) 100 80 60 Nintedanib + docetaxel Placebo + docetaxel Median, mo 3. 4 2. 7 HR (95% CI) 0. 79 (0. 68 to 0. 92) p-value 0. 0019 40 20 0 0 2 4 No. at risk Nintedanib 565 295 155 Placebo 569 250 116 43 6 8 57 21 10 12 14 Time (months) 19 2 4 1 3 0 16 1 0 18 0

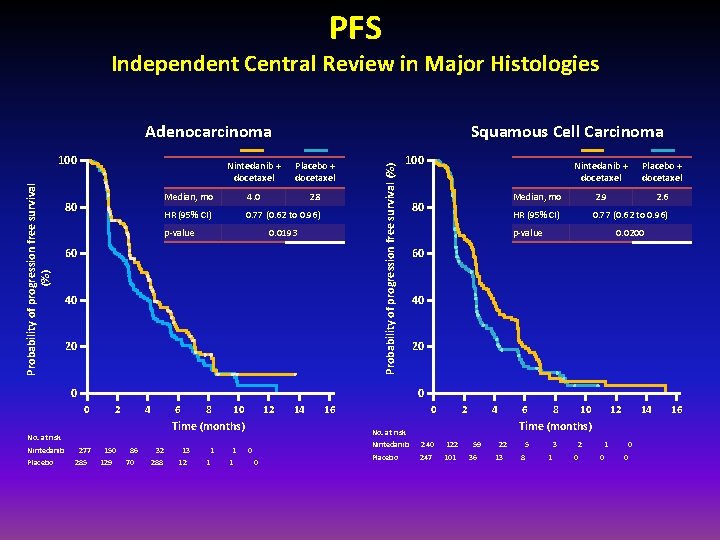
PFS Independent Central Review in Major Histologies Probability of progression free survival (%) 100 80 Squamous Cell Carcinoma Nintedanib + docetaxel Placebo + docetaxel Median, mo 4. 0 2. 8 HR (95% CI) 0. 77 (0. 62 to 0. 96) p-value 0. 0193 60 40 20 Probability of progression free survival (%) Adenocarcinoma 100 0 2 4 6 8 10 12 Time (months) No. at risk Placebo + docetaxel Median, mo 2. 9 2. 6 HR (95% CI) 0. 77 (0. 62 to 0. 96) p-value 0. 0200 60 40 20 0 0 Nintedanib 80 Nintedanib + docetaxel 277 285 150 129 86 70 32 288 13 12 1 1 14 16 0 2 4 6 0 10 12 14 Time (months) No. at risk 0 8 Nintedanib 240 122 Placebo 247 101 59 36 22 13 5 8 3 1 2 0 1 0 0 0 16

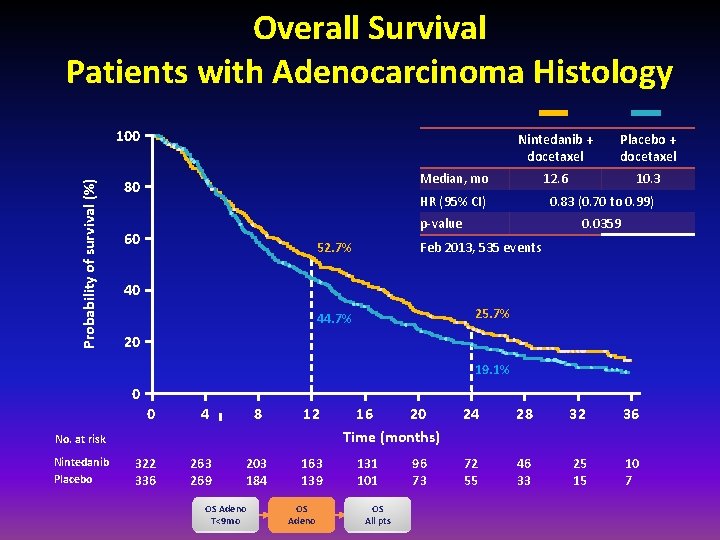
Overall Survival Patients with Adenocarcinoma Histology Probability of survival (%) 100 Nintedanib + docetaxel Placebo + docetaxel 12. 6 10. 3 Median, mo 80 HR (95% CI) 0. 83 (0. 70 to 0. 99) p-value 60 52. 7% 0. 0359 Feb 2013, 535 events 40 25. 7% 44. 7% 20 19. 1% 0 0 4 8 12 322 336 263 269 203 184 163 139 No. at risk Nintedanib Placebo OS Adeno T<9 mo OS Adeno 16 20 Time (months) 131 101 OS All pts 96 73 24 28 32 36 72 55 46 33 25 15 10 7

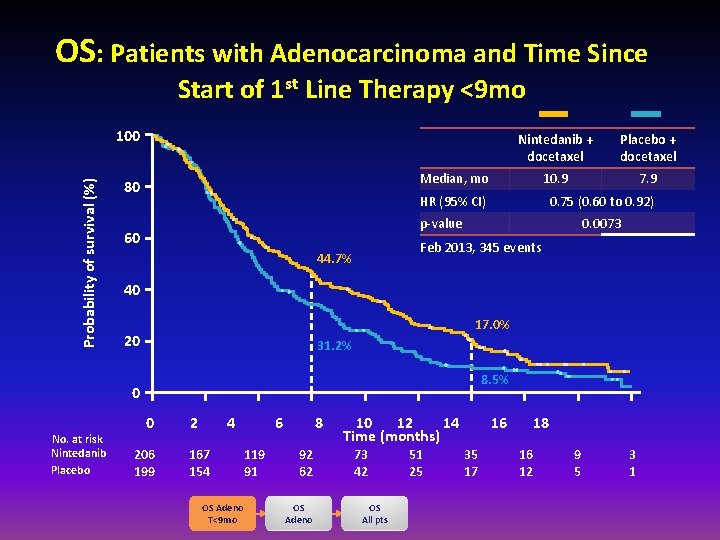
OS: Patients with Adenocarcinoma and Time Since Start of 1 st Line Therapy <9 mo Probability of survival (%) 100 Placebo + docetaxel 10. 9 7. 9 Median, mo 80 HR (95% CI) 0. 75 (0. 60 to 0. 92) p-value 60 0. 0073 Feb 2013, 345 events 44. 7% 40 17. 0% 20 31. 2% 8. 5% 0 No. at risk Nintedanib Placebo Nintedanib + docetaxel 0 206 199 2 4 167 154 OS Adeno T<9 mo 6 119 91 8 92 62 OS Adeno 10 12 14 Time (months) 73 42 OS All pts 51 25 16 35 17 18 16 12 9 5 3 1

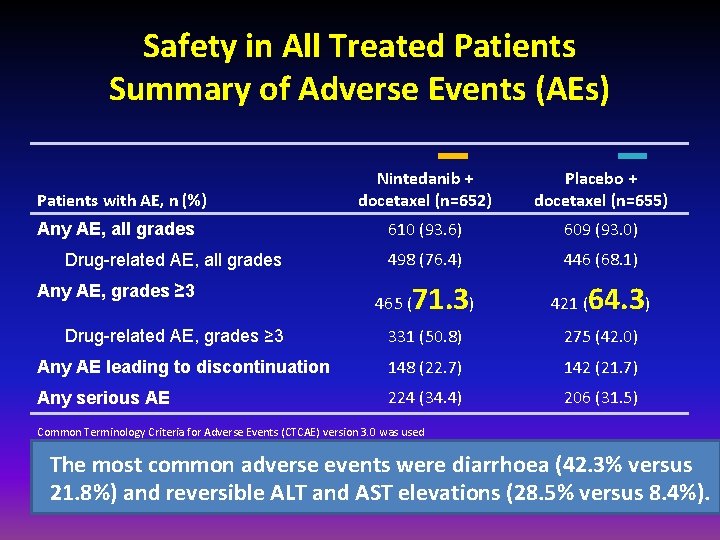
Safety in All Treated Patients Summary of Adverse Events (AEs) Patients with AE, n (%) Any AE, all grades Drug-related AE, all grades Any AE, grades ≥ 3 Nintedanib + docetaxel (n=652) Placebo + docetaxel (n=655) 610 (93. 6) 609 (93. 0) 498 (76. 4) 446 (68. 1) 465 ( 71. 3) 421 ( 64. 3) 331 (50. 8) 275 (42. 0) Any AE leading to discontinuation 148 (22. 7) 142 (21. 7) Any serious AE 224 (34. 4) 206 (31. 5) Drug-related AE, grades ≥ 3 Common Terminology Criteria for Adverse Events (CTCAE) version 3. 0 was used The most common adverse events were diarrhoea (42. 3% versus 21. 8%) and reversible ALT and AST elevations (28. 5% versus 8. 4%).

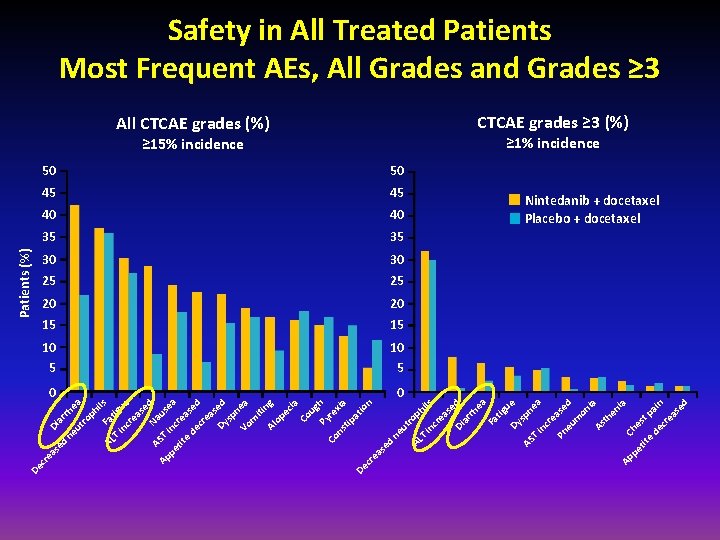
in ue as cr e ils AS e Na d u Ap T pe inc sea r tit e eas de e cr d ea s Dy ed sp n Vo ea m iti Al ng op ec ia Co ug Py h re Co ns xia tip at De io n cr ea se d ne ut AL rop hi T ls in cr ea se d Di ar rh ea Fa tig u Dy e sp AS ne T a in cr e Pn ase eu d m on ia As th en ia Ap pe Che s tit e t pa de i cr n ea se d T tig Fa ea ph ro rh ar Di ut ne AL ed as cr e De Patients (%) Safety in All Treated Patients Most Frequent AEs, All Grades and Grades ≥ 3 All CTCAE grades (%) CTCAE grades ≥ 3 (%) ≥ 15% incidence ≥ 1% incidence 50 50 45 45 40 40 35 35 30 30 25 25 20 20 15 15 10 5 0 0 Nintedanib + docetaxel Placebo + docetaxel

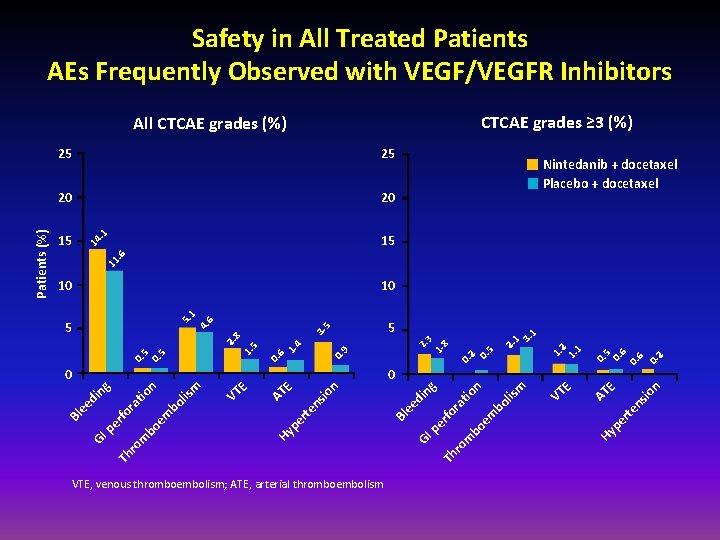
Safety in All Treated Patients AEs Frequently Observed with VEGF/VEGFR Inhibitors CTCAE grades ≥ 3 (%) 25 20 20 15 15 Nintedanib + docetaxel Placebo + docetaxel 11 . 6 14 . 1 25 0. 6 0. 2 io n E er te ns AT VT E m lis bo em bo m ro Th GI pe rfo ra tio n ng di ee Bl io ns te er VTE, venous thromboembolism; ATE, arterial thromboembolism Hy p 0. 2 0. 5 0. 9 n E VT E AT Hy p em bo lis n tio bo m ro Th GI pe rfo ra di ee m 0 ng 0 2. 1 3. 1 5 2. 3 1. 8 3. 5 0. 6 1. 4 0. 5 1. 5 2. 8 5 1. 2 1. 1 10 5. 1 4. 6 10 Bl Patients (%) All CTCAE grades (%)

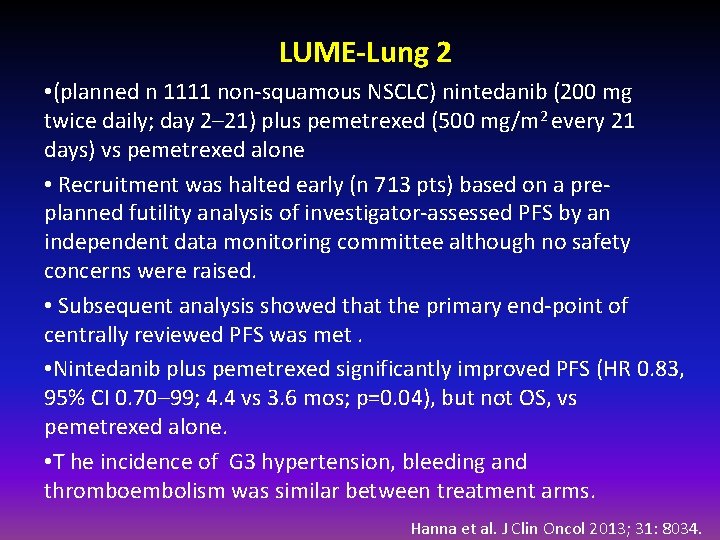
LUME-Lung 2 • (planned n 1111 non-squamous NSCLC) nintedanib (200 mg twice daily; day 2– 21) plus pemetrexed (500 mg/m 2 every 21 days) vs pemetrexed alone • Recruitment was halted early (n 713 pts) based on a preplanned futility analysis of investigator-assessed PFS by an independent data monitoring committee although no safety concerns were raised. • Subsequent analysis showed that the primary end-point of centrally reviewed PFS was met. • Nintedanib plus pemetrexed significantly improved PFS (HR 0. 83, 95% CI 0. 70– 99; 4. 4 vs 3. 6 mos; p=0. 04), but not OS, vs pemetrexed alone. • T he incidence of G 3 hypertension, bleeding and thromboembolism was similar between treatment arms. Hanna et al. J Clin Oncol 2013; 31: 8034.

Outline • Antiangiogenic agents in the first-line setting • Bevacizumab • Recently completed randomised trials • Multi-targeted antiangiogenic orally administered TKIs • Vascular disrupting agents • Antiangiogenic agents in a relapsed/refractory setting • Recently completed randomised trials • Multi-targeted antiangiogenic orally administered TKIs -Nindetanib • Monoclonal antibodies and decoy receptors • Vascular disrupting agents • Other investigational agents • Ramucirumab

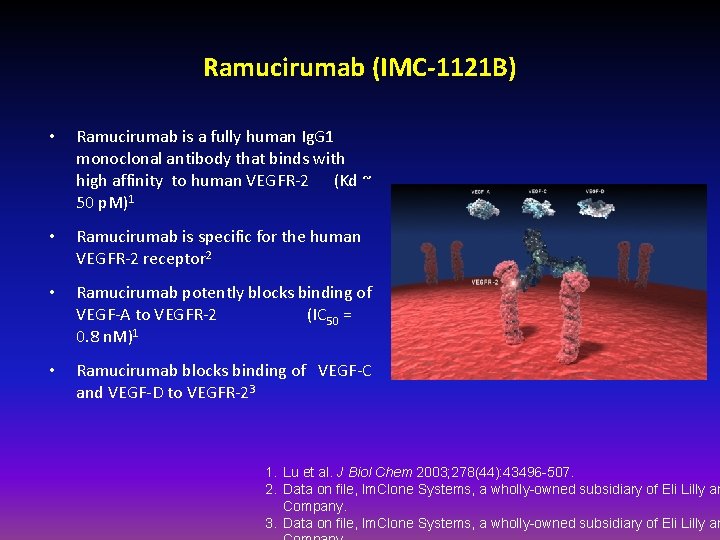
Ramucirumab (IMC-1121 B) • Ramucirumab is a fully human Ig. G 1 monoclonal antibody that binds with high affinity to human VEGFR-2 (Kd ~ 50 p. M)1 • Ramucirumab is specific for the human VEGFR-2 receptor 2 • Ramucirumab potently blocks binding of VEGF-A to VEGFR-2 (IC 50 = 0. 8 n. M)1 • Ramucirumab blocks binding of VEGF-C and VEGF-D to VEGFR-23 1. Lu et al. J Biol Chem 2003; 278(44): 43496 -507. 2. Data on file, Im. Clone Systems, a wholly-owned subsidiary of Eli Lilly an Company. 3. Data on file, Im. Clone Systems, a wholly-owned subsidiary of Eli Lilly an

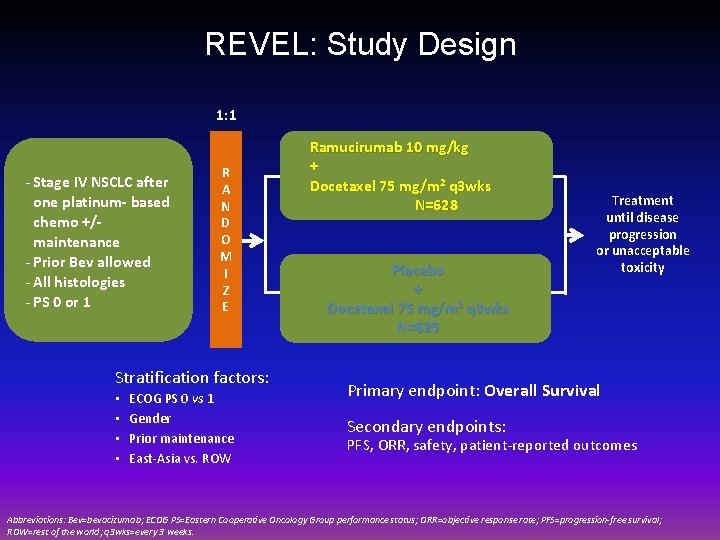
REVEL: Study Design 1: 1 - Stage IV NSCLC after one platinum- based chemo +/maintenance - Prior Bev allowed - All histologies - PS 0 or 1 R A N D O M I Z E Stratification factors: • • ECOG PS 0 vs 1 Gender Prior maintenance East-Asia vs. ROW Ramucirumab 10 mg/kg + Docetaxel 75 mg/m 2 q 3 wks N=628 Placebo + Docetaxel 75 mg/m 2 q 3 wks N=625 Treatment until disease progression or unacceptable toxicity Primary endpoint: Overall Survival Secondary endpoints: PFS, ORR, safety, patient-reported outcomes Abbreviations: Bev=bevacizumab; ECOG PS=Eastern Cooperative Oncology Group performance status; ORR=objective response rate; PFS=progression-free survival; ROW=rest of the world; q 3 wks=every 3 weeks.

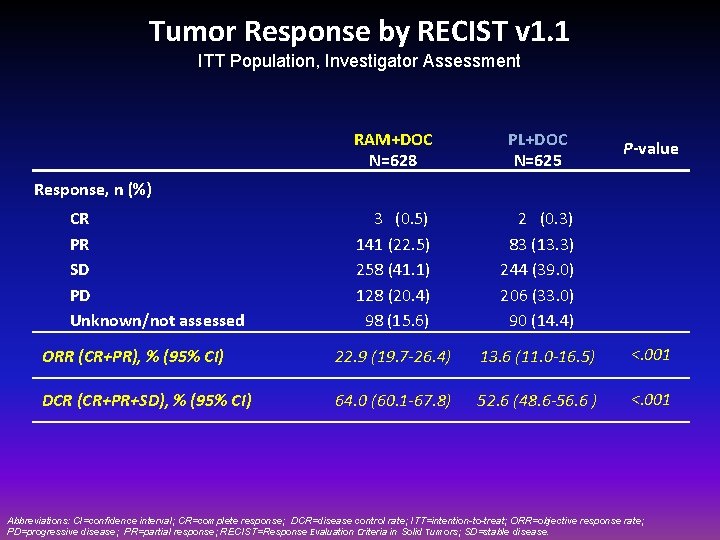
Tumor Response by RECIST v 1. 1 ITT Population, Investigator Assessment RAM+DOC N=628 PL+DOC N=625 3 (0. 5) 141 (22. 5) 258 (41. 1) 128 (20. 4) 98 (15. 6) 2 (0. 3) 83 (13. 3) 244 (39. 0) 206 (33. 0) 90 (14. 4) ORR (CR+PR), % (95% CI) 22. 9 (19. 7 -26. 4) 13. 6 (11. 0 -16. 5) <. 001 DCR (CR+PR+SD), % (95% CI) 64. 0 (60. 1 -67. 8) 52. 6 (48. 6 -56. 6 ) <. 001 P-value Response, n (%) CR PR SD PD Unknown/not assessed Abbreviations: CI=confidence interval; CR=complete response; DCR=disease control rate; ITT=intention-to-treat; ORR=objective response rate; PD=progressive disease; PR=partial response; RECIST=Response Evaluation Criteria in Solid Tumors; SD=stable disease.

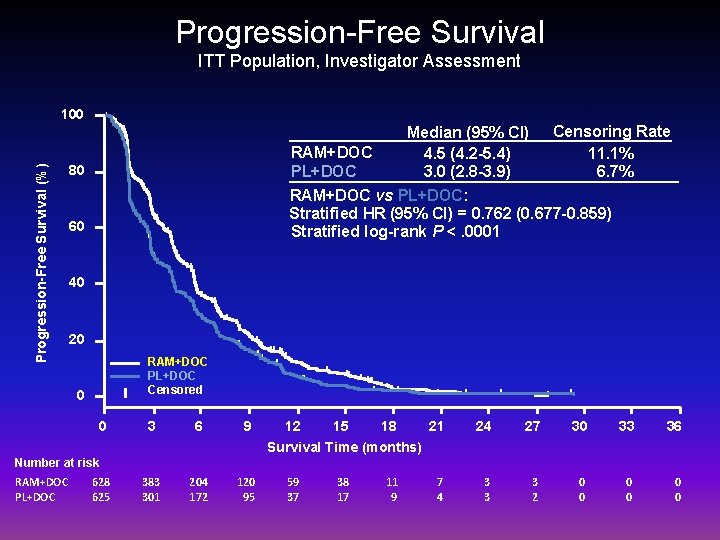
Progression-Free Survival ITT Population, Investigator Assessment Progression-Free Survival (%) 100 Median (95% CI) Censoring Rate RAM+DOC 4. 5 (4. 2 -5. 4) 11. 1% PL+DOC 3. 0 (2. 8 -3. 9) 6. 7% RAM+DOC vs PL+DOC: Stratified HR (95% CI) = 0. 762 (0. 677 -0. 859) Stratified log-rank P <. 0001 80 60 40 20 RAM+DOC PL+DOC Censored 0 0 3 6 9 12 15 18 21 24 27 30 33 36 7 4 3 3 3 2 0 0 0 Survival Time (months) Number at risk RAM+DOC PL+DOC 628 625 383 301 204 172 120 95 59 37 38 17 11 9

Forest Plot of PFS by Subgroups Unstratified Analysis Category Subgroup RAM+DOC, n PL+DOC, n Unstratified HR Geographic region ROW East Asia Male Female ≥ 70 <70 1 0 Never Ever PD CR/PR/SD No 585 43 419 209 127 501 420 207 109 518 178 420 475 579 46 415 210 125 500 425 199 141 483 182 417 476 0. 78 0. 68 0. 79 0. 74 0. 94 0. 73 0. 79 0. 74 0. 81 0. 76 0. 71 0. 80 0. 74 Yes No 153 540 88 493 149 533 92 482 0. 90 0. 77 0. 83 0. 79 Yes Squamous Nonsquamous 135 157 465 143 171 447 0. 74 0. 76 0. 77 Sex Age, years ECOG PS Smoking history Best response to platinum Prior taxane Prior bevacizumab Prior maintenance Histology 0. 3 0. 6 Favors RAM+DOC 1. 0 1. 6 2. 7 4. 5 Favors PL+DOC

Overall Survival ITT Population 100 RAM+DOC PL+DOC Overall Survival (%) 80 Censoring Rate 31. 8% 27. 0% Median (95% CI) 10. 5 (9. 5 -11. 2) 9. 1 (8. 4 -10. 0) RAM+DOC vs PL+DOC: Stratified HR (95% CI) = 0. 857 (0. 751 -0. 979) Stratified log-rank P =. 0235 60 40 20 RAM+DOC PL+DOC Censored 0 0 3 6 9 12 18 21 24 27 30 33 36 45 36 23 23 11 9 2 0 0 0 Survival Time (months) Number at risk RAM+DOC 628 PL+DOC 625 15 527 501 415 386 329 306 231 197 156 129 103 86 70 56

REVEL: Conclusions • REVEL met its primary endpoint of OS improvement. • RAM+DOC showed statistically significant improvement in PFS and ORR compared to PL+DOC. • OS and PFS improvement were consistent in most major subgroups, including squamous and nonsquamous histology. • The addition of RAM to DOC did not result in an increase of SAEs and AEs leading to death. Safety profile was as expected for an anti-VEGFR agent in combination with DOC. • REVEL is the first study showing that addition of a novel agent to standard chemotherapy improves survival in stage IV NSCLC patients with progression after platinum-based chemotherapy.

Predictive markers antiangiogenic agents • Imaging • PET • Dynamic Contrast enhanced (DCE) MRI • Alternative RECIST criteria • • Physiologic: hypertension Circulating endothelial cells VEGF polymorphisms Circulating vascular Marker

Take home messages • Why there has been such an high failure rate of trials of antiangiogenic agents in NSCLC? - agents may not effectively combat the redundancy of angiogenic pathways. - highly heterogeneous nature of NSCLC • Confirmed efficacy of bevacizumab in eligible patients in first-line (non-squamous, no invasion of central blood vessels, no history of gross hemoptysis, no major cardiovascular disease) • Nindetanib and Ramucirumab might be important news in the NSCLC scenario in the second line setting • Currently no predictive biomarkers available
 Sprouting and intussusceptive angiogenesis
Sprouting and intussusceptive angiogenesis Tumor angiogenesis
Tumor angiogenesis Angiogenesis
Angiogenesis Servier medical art
Servier medical art Optimal lung cancer pathway
Optimal lung cancer pathway Tnm staging lung cancer
Tnm staging lung cancer Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Lifetime risk of lung cancer
Lifetime risk of lung cancer Siadh pathophysiology
Siadh pathophysiology Actual diagnosis
Actual diagnosis Gesundheit central european lung cancer patient network
Gesundheit central european lung cancer patient network Dr. amy ford
Dr. amy ford Quando fra l'altre donne ad ora ad ora
Quando fra l'altre donne ad ora ad ora Surveillance drain de redon
Surveillance drain de redon Ramon lucio park
Ramon lucio park Quadrifarmaco
Quadrifarmaco Liceo classico lucio anneo seneca
Liceo classico lucio anneo seneca Lucio rossi
Lucio rossi Lucio accio
Lucio accio Lucio tarquinio
Lucio tarquinio Lucio andreani
Lucio andreani Samuel krause
Samuel krause Lucio rossi
Lucio rossi Giovanni allegro
Giovanni allegro Le défunt par erreur
Le défunt par erreur A me me piace a nutella
A me me piace a nutella Funciones de los elementos
Funciones de los elementos Robert lucio
Robert lucio No es cierto que paco y daniel nos 1 of 1 (ayudar).
No es cierto que paco y daniel nos 1 of 1 (ayudar). Target marketing
Target marketing Alcohol commercials targeting youth
Alcohol commercials targeting youth Concentrated marketing
Concentrated marketing Market segmentation and strategic targeting
Market segmentation and strategic targeting Segmentace targeting positioning
Segmentace targeting positioning Market segmentation, targeting and positioning
Market segmentation, targeting and positioning Https://www.census.gov/popclock/
Https://www.census.gov/popclock/ Undifferentiated targeting strategy
Undifferentiated targeting strategy Joint targeting cycle
Joint targeting cycle