Subatomic Particles and Quantum Theory Atomic Physics Subatomic

Subatomic Particles and Quantum Theory Atomic Physics

Subatomic Particle Tracks Were originally detected by Charles Thompson Rees in 1910 using a cloud chamber A cloud chamber is a confined space super saturated with water or alcohol vapour high energy particles moving though them condense the droplets and produce a vapour trail

Charged Particles Only particles that have a charge create tracks of condensed liquid in the cloud chamber http: //www. youtube. com/watch? v=m. LDASjzbxl. A&feature=related http: //www. youtube. com/watch? v=kuz. WNOUq. Lm. Q&feature=related


Uses One of the earliest ways of detecting the path of charged particles. Could be placed in magnetic fields to study path. Principle tool of particle physics for 50 years.

Bubble Chamber Contains a liquefied gas (ie. H 2, He, Xe) Lowering the pressure reduces the boiling pt to just below actual temperature. Charged particles passing through cause liquid to boil, leaving a trail of bubbles. http: //www. youtube. com/watch? v=qc. Uw. LH 8 L 5 AU
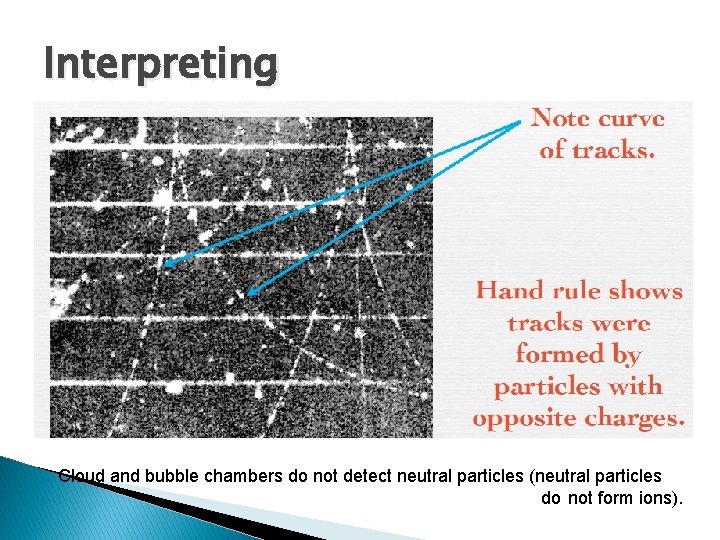
Interpreting Cloud and bubble chambers do not detect neutral particles (neutral particles do not form ions).

In a bubble chamber, these particles create a series of bubbles in the trail -q +q Right hand rule � Particle . . B directed out of the plane of the page tracks are used to determine the radius of the path of a charged particle or q/m ratio

Spiral Tracks The spiral results from the charged particle slowing in the bubble chamber. r decreases as v decreases
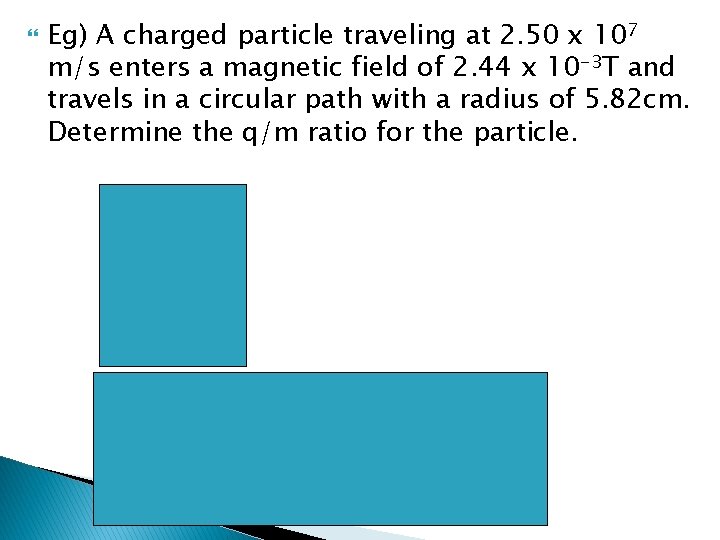
Eg) A charged particle traveling at 2. 50 x 107 m/s enters a magnetic field of 2. 44 x 10 -3 T and travels in a circular path with a radius of 5. 82 cm. Determine the q/m ratio for the particle.
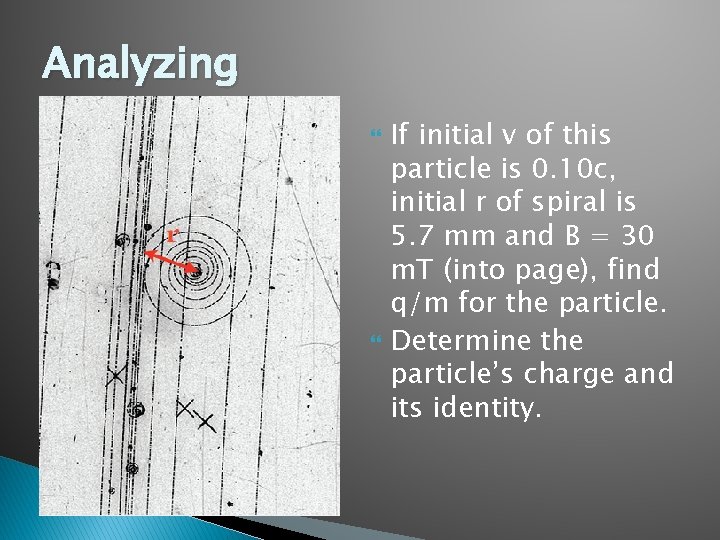
Analyzing If initial v of this particle is 0. 10 c, initial r of spiral is 5. 7 mm and B = 30 m. T (into page), find q/m for the particle. Determine the particle’s charge and its identity.
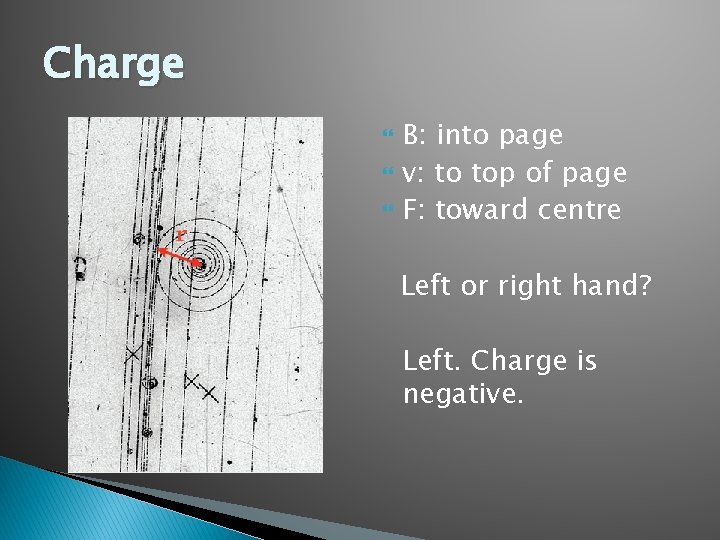
Charge B: into page v: to top of page F: toward centre Left or right hand? Left. Charge is negative.
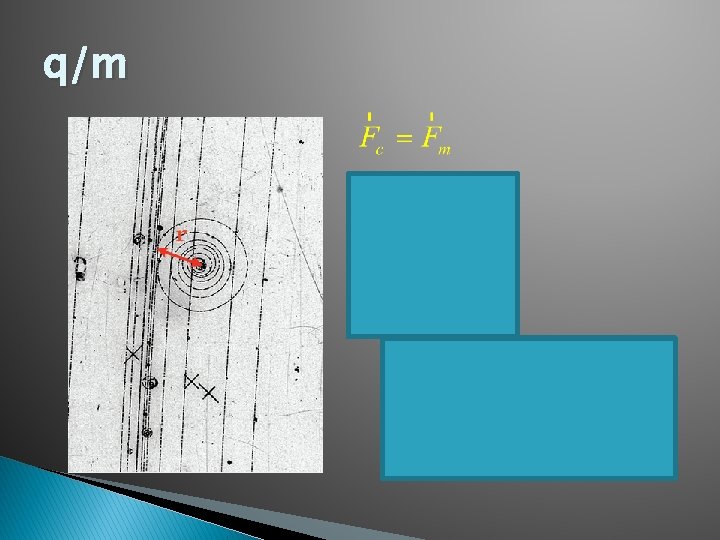
q/m
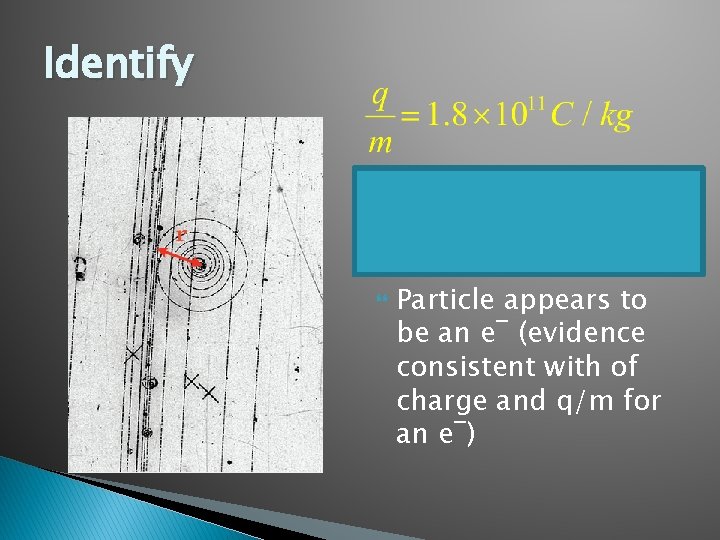
Identify Particle appears to be an e¯ (evidence consistent with of charge and q/m for an e¯)

Collisions Collisions can be seen when two tracks emerge from one. Collisions with a neutral particle (that leave no track) appear to emerge from nowhere. This is something different.
Antimatter • • early 1900’s, 3 fundamental particles: electrons, protons, and neutron fundamental particle – particle that cannot be split or divided into smaller pieces Carl Anderson, 1923, discovered antimatter where a particle track in a cloud chamber had mass of an electron but opposite charge the collision of a particle (e-) and antiparticle (e+) can annihilate both particles and create a pair of high energy gamma rays traveling in opposite directions
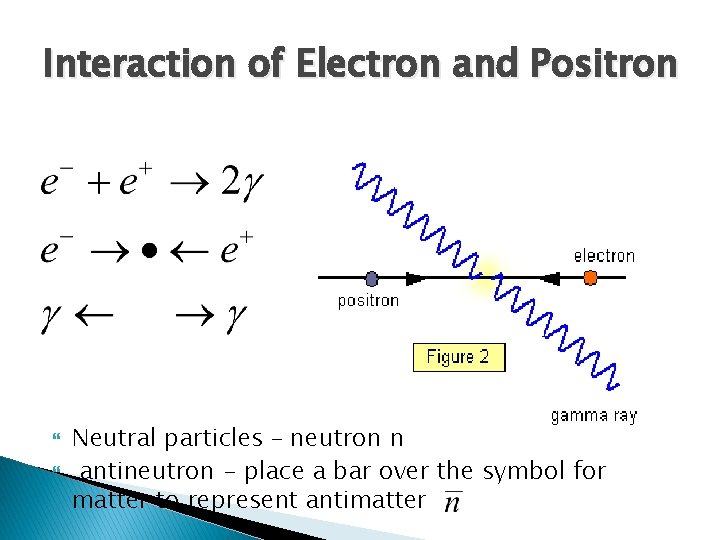
Interaction of Electron and Positron Neutral particles – neutron n antineutron - place a bar over the symbol for matter to represent antimatter
Eg) PET (positron emission topography) Electron - positron annihilation fluorine-18 emits positrons as it decays, these collide with electrons in body cells and the gamma rays are detected showing the concentration of the radioactive tracer element injected into the body
Quantum Theory Quantum theory describes mediating particles that exist to indicate that fundamental forces act on particles over some distance Particles that mediate forces for a brief time are virtual particles, energy, mass, momentum, etc. are not the same as real particles
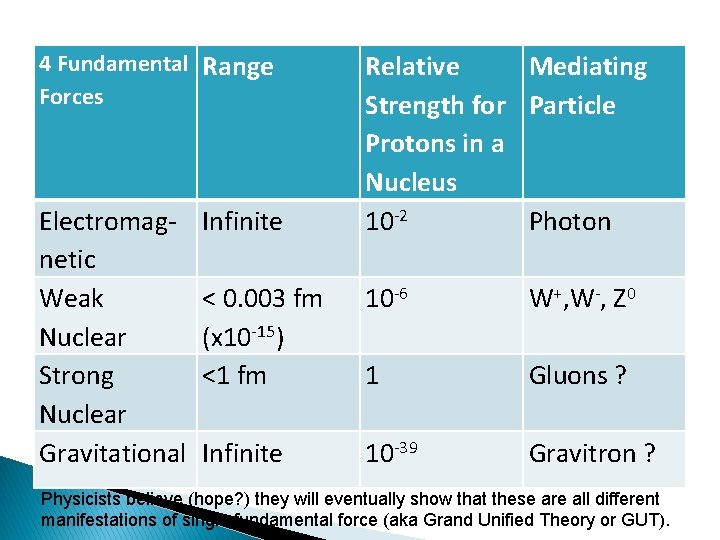
4 Fundamental Range Forces Electromagnetic Weak Nuclear Strong Nuclear Gravitational Infinite Relative Mediating Strength for Particle Protons in a Nucleus 10 -2 Photon < 0. 003 fm (x 10 -15) <1 fm 10 -6 W+, W-, Z 0 1 Gluons ? Infinite 10 -39 Gravitron ? Physicists believe (hope? ) they will eventually show that these are all different manifestations of single fundamental force (aka Grand Unified Theory or GUT).

strong nuclear force is very large over a short distance (x 10 -8 m) which is the size of a nucleus - to separate the parts of the nucleus, scientists need very large energies cosmic rays – energies of 102 – 1014 Me. V - are EM photons protons, - antiprotons, electrons positrons, alpha particles

Subatomic Particles ( ~ 300) Muons – unstable particles having similar properties to electrons but 207 x the mass of the electron Pion – unstable particle – 270 x mass of the electron

The Large Hadron Collider at CERN can accelerate protons to an energy of about 7 Te. V (7 x 1012 e. V) each, or lead nuclei to about 574 Te. V per nucleus. Beams of protons, moving in opposite directions, will be collided (each beam moving very close to c). http: //www. youtube. com/watch? v=j 50 Zss. Eojt. M CERN on Wikipedia
Two Separate Families Leptons – subatomic particles that do not interact by means of a strong nuclear force eg) electrons, neutrinos, muons Hadrons – subatomic particles that do interact by means of a strong nuclear force eg) protons and neutrons

Hadrons are divided into 2 groups based upon spin 1. Mesons – hadron with an integer spin eg(0, 1, or 2) eg) pion 2. Baryons- hadron with a half integer spin eg (1/2, 3/2) eg) proton or neutron Text: p. 842 – 843 tables *Fermion – a particle with a half integer spin – all leptons and baryons Boson – a particle with an integer spin
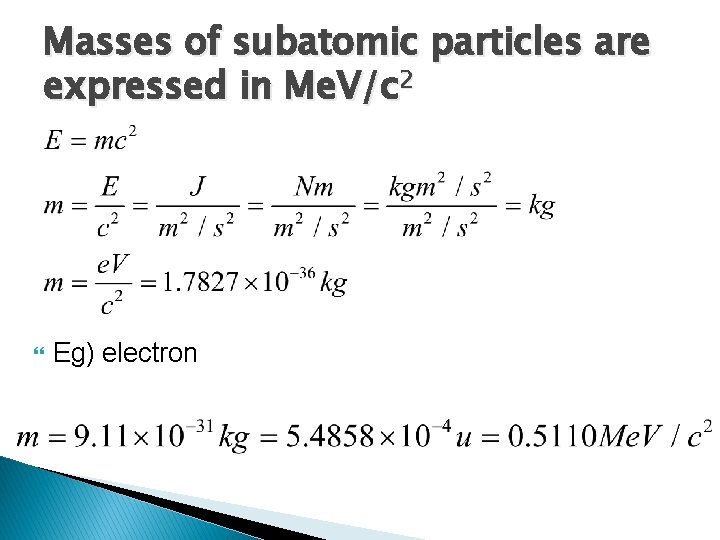
Masses of subatomic particles are expressed in Me. V/c 2 Eg) electron

Proton
- Slides: 27