STOICHIOMETRY A recipe for chemistry Step 1 Stoichiometry






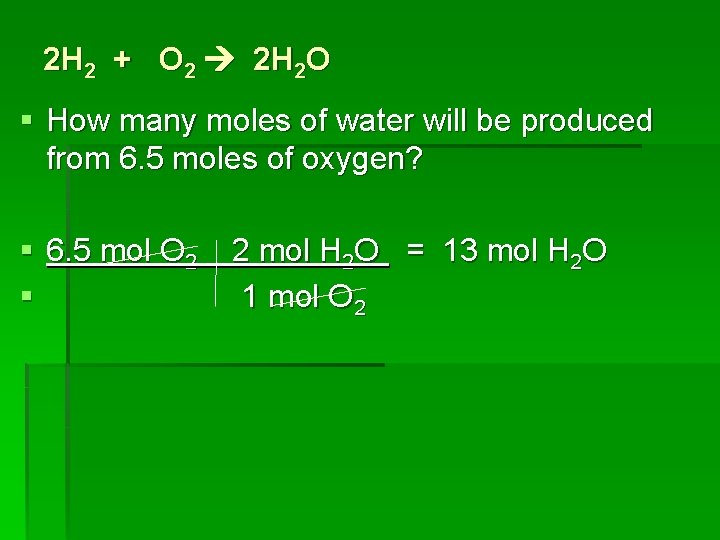
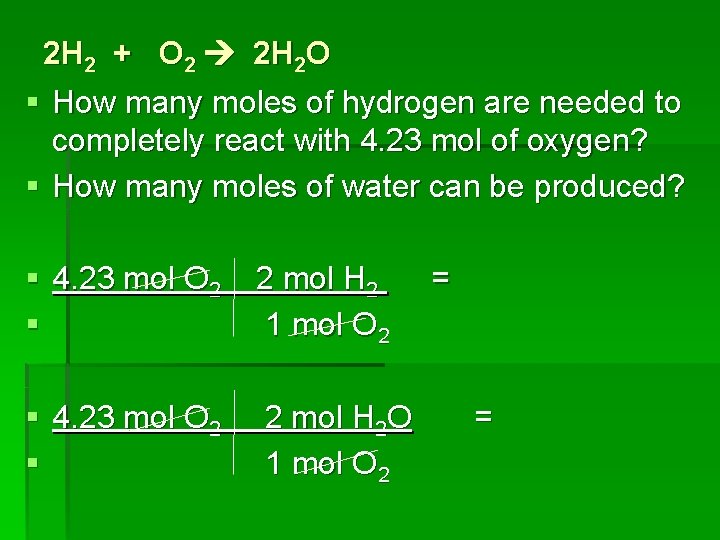
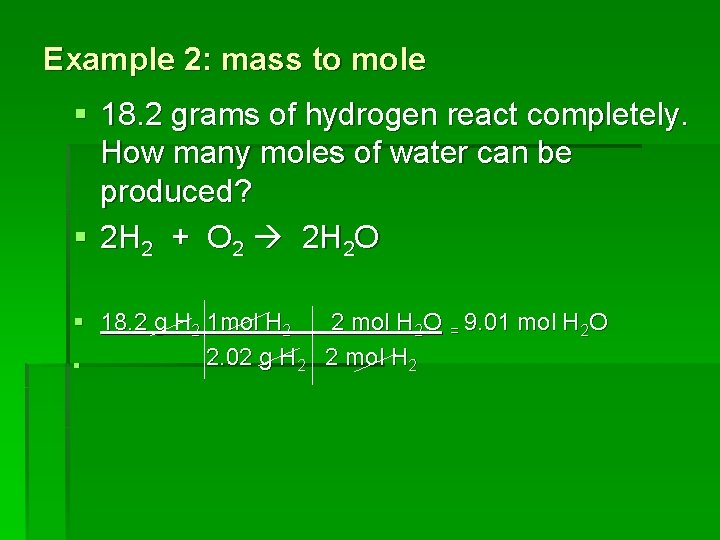





- Slides: 17

STOICHIOMETRY A recipe for chemistry!


Step 1 § Stoichiometry begins with a balanced chemical equation. § This means that formulas must be written correctly and you may even have to predict the products!!

Step 2 § Use ratios from the BCE to determine the amount of reactants needed or the amount of products formed from the given substance and quantity.

Step 3 § If quantity is given in moles, go directly to the mole ratio from the BCE. § If given grams, convert to moles before using the mole ratio from the BCE.

Step 4 § Check to make sure answer is in the correct form (unit) that was asked for in the problem.

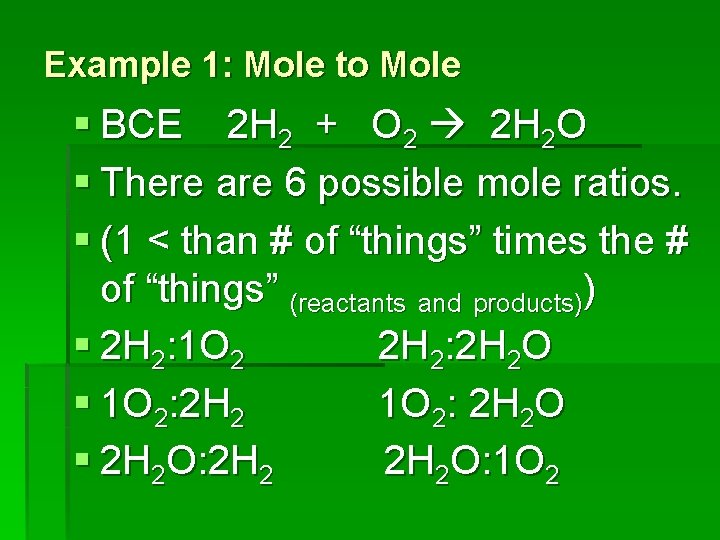
Example 1: Mole to Mole § BCE 2 H 2 + O 2 2 H 2 O § There are 6 possible mole ratios. § (1 < than # of “things” times the # of “things” (reactants and products)) § 2 H 2: 1 O 2 2 H 2: 2 H 2 O § 1 O 2: 2 H 2 O § 2 H 2 O: 1 O 2
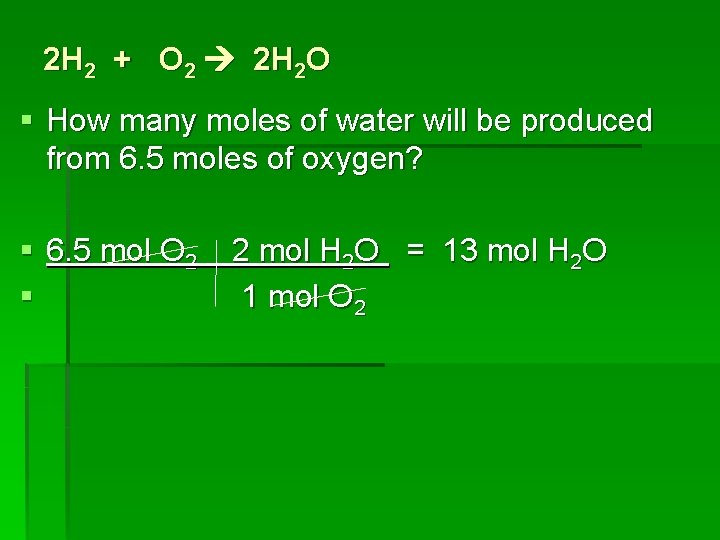
2 H 2 + O 2 2 H 2 O § How many moles of water will be produced from 6. 5 moles of oxygen? § 6. 5 mol O 2 § 2 mol H 2 O = 13 mol H 2 O 1 mol O 2
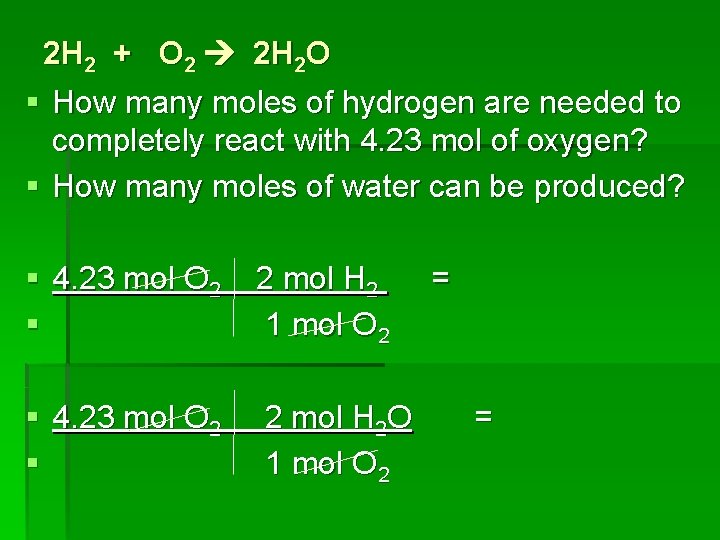
2 H 2 + O 2 2 H 2 O § How many moles of hydrogen are needed to completely react with 4. 23 mol of oxygen? § How many moles of water can be produced? § 4. 23 mol O 2 § 2 mol H 2 1 mol O 2 § 4. 23 mol O 2 § 2 mol H 2 O 1 mol O 2 = =
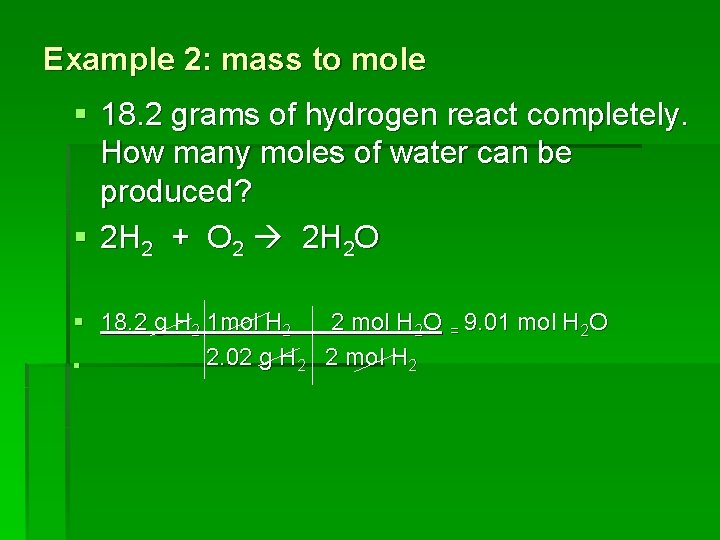
Example 2: mass to mole § 18. 2 grams of hydrogen react completely. How many moles of water can be produced? § 2 H 2 + O 2 2 H 2 O § 18. 2 g H 2 1 mol H 2 2 mol H 2 O = 9. 01 mol H 2 O 2. 02 g H 2 2 mol H 2 §

§ 2 Na(s) + Cl 2(g) 2 Na. Cl(s) How many moles of sodium are needed to produce 2. 4 grams of sodium chloride? 2. 4 g. Na. Cl 1 mol Na. Cl 2 mol Na = 58. 44 g Na. Cl 2 mol Na. Cl

2 Na(s) + Cl 2(g) 2 Na. Cl(s) § How many moles of chlorine are needed to produce 2. 4 grams of sodium chloride? § 2. 4 g Na. Cl 1 mol Cl 2 __ = § 58. 44 g Na. Cl 2 mol Na. Cl

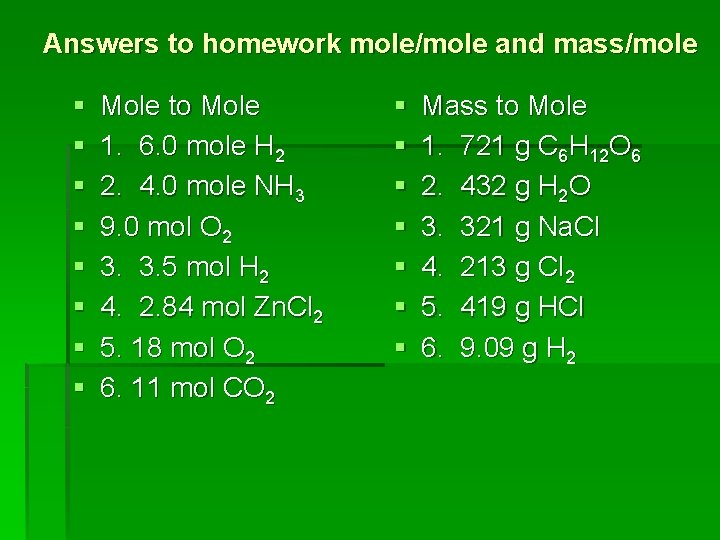
Answers to homework mole/mole and mass/mole § § § § Mole to Mole 1. 6. 0 mole H 2 2. 4. 0 mole NH 3 9. 0 mol O 2 3. 3. 5 mol H 2 4. 2. 84 mol Zn. Cl 2 5. 18 mol O 2 6. 11 mol CO 2 § § § § Mass to Mole 1. 721 g C 6 H 12 O 6 2. 432 g H 2 O 3. 321 g Na. Cl 4. 213 g Cl 2 5. 419 g HCl 6. 9. 09 g H 2


Example 3: mass to mass § 4 NH 3 + 5 O 2 4 NO + 6 H 2 O How many grams of water can be produced from 15. 6 grams of ammonia? 15. 6 g NH 3 1 mol NH 3 6 mol H 2 O 17. 04 g NH 3 4 mol NH 3 = 18. 02 g H 2 O 1 mol H 2 O

4 NH 3 + 5 O 2 4 NO + 6 H 2 O § How many grams of oxygen are needed to produce 21. 7 grams of NO? 21. 7 g NO 1 mol NO 5 mol O 2 30. 01 g NO 4 mol NO = 32. 00 g O 2 1 mol O 2

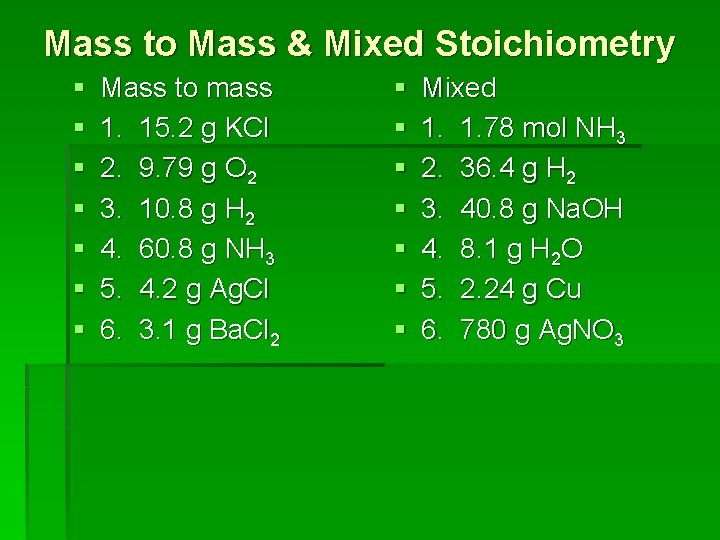
Mass to Mass & Mixed Stoichiometry § § § § Mass to mass 1. 15. 2 g KCl 2. 9. 79 g O 2 3. 10. 8 g H 2 4. 60. 8 g NH 3 5. 4. 2 g Ag. Cl 6. 3. 1 g Ba. Cl 2 § § § § Mixed 1. 1. 78 mol NH 3 2. 36. 4 g H 2 3. 40. 8 g Na. OH 4. 8. 1 g H 2 O 5. 2. 24 g Cu 6. 780 g Ag. NO 3
 Step 1 step 2 step 3 step 4
Step 1 step 2 step 3 step 4 What is the first step in most stoichiometry problems?
What is the first step in most stoichiometry problems? Unit: stoichiometry “multi-step problems” – ws #3
Unit: stoichiometry “multi-step problems” – ws #3 Chapter 11 stoichiometry answer key
Chapter 11 stoichiometry answer key Chapter 9 modern chemistry review answers
Chapter 9 modern chemistry review answers A balanced chemical equation allows one to determine the
A balanced chemical equation allows one to determine the Ap chemistry stoichiometry
Ap chemistry stoichiometry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry Stoichiometry is
Stoichiometry is Ap chemistry stoichiometry
Ap chemistry stoichiometry Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Step-by step inventory process
Step-by step inventory process Dimensional analysis steps
Dimensional analysis steps Jamie tried to solve an equation step by step.
Jamie tried to solve an equation step by step. Steps to writing an argumentative essay
Steps to writing an argumentative essay Portfolio management process
Portfolio management process Step by step punnett square
Step by step punnett square Two step inequalities with fractions
Two step inequalities with fractions