Solutions Solutions Classification are homogeneous mixtures of Matter






- Slides: 24

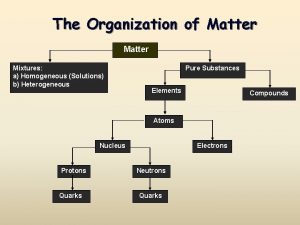
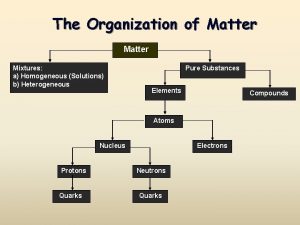
Solutions

Solutions Classification are homogeneous mixtures of Matter

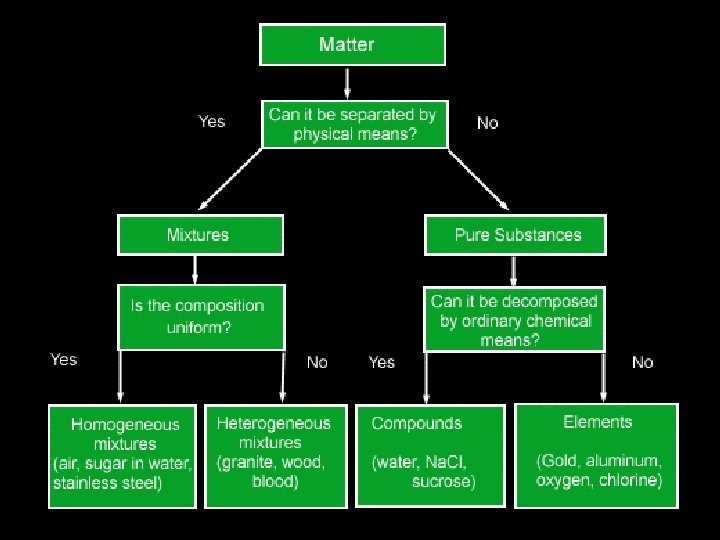
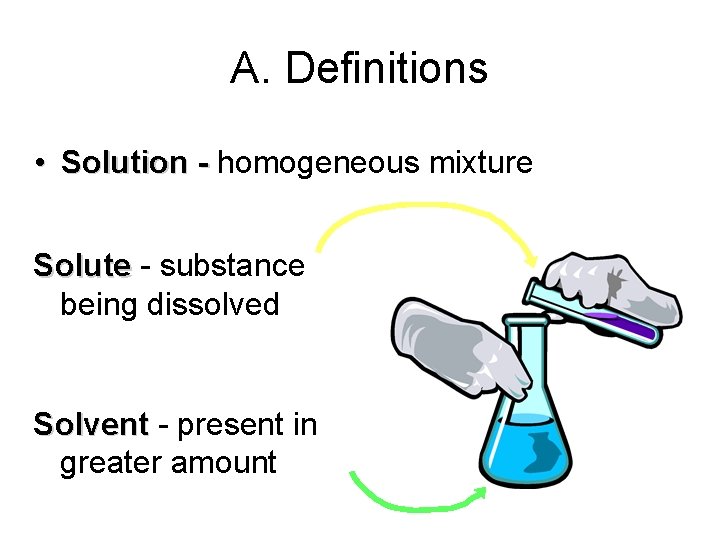
A. Definitions • Solution - homogeneous mixture Solute - substance being dissolved Solvent - present in greater amount

Solute A solute is the dissolved substance in a solution. Salt in salt water Solvent Sugar in soda drinks Carbon dioxide in soda drinks A solvent is the dissolving medium in a solution. Water in salt water Water in soda

Soluble and Insoluble n n Soluble: when a solute dissolves in a solvent Insoluble: When a solute doesn’t dissolve in a solvent Miscible: When a liquid dissolves in another liquid Immiscible: When 2 liquids don’t dissolve


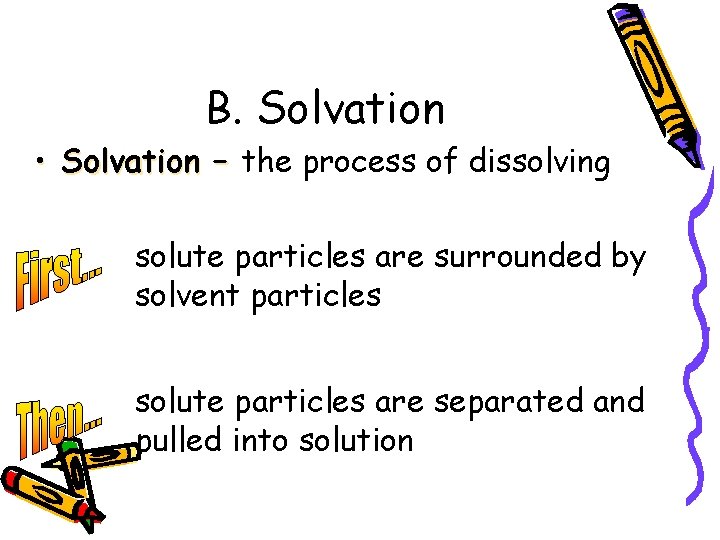
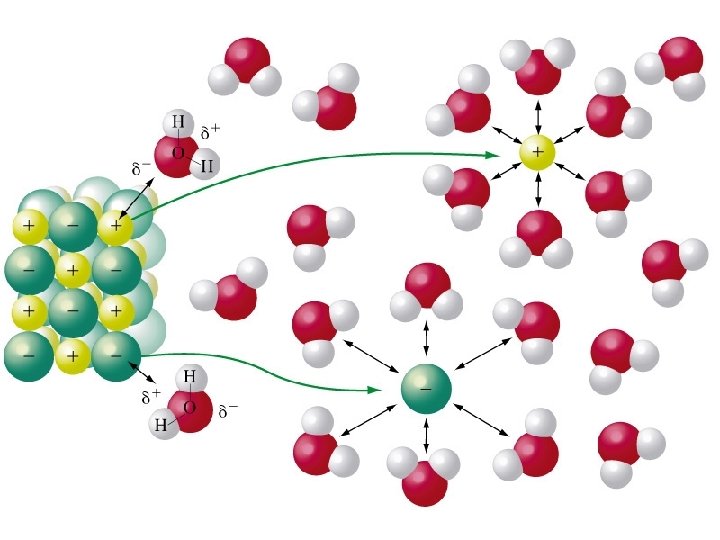
B. Solvation • Solvation – the process of dissolving solute particles are surrounded by solvent particles solute particles are separated and pulled into solution

Solvents at the hardware store

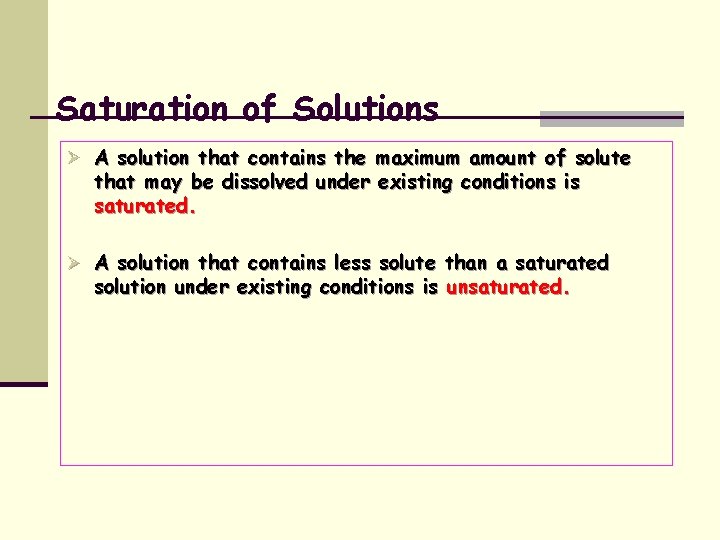
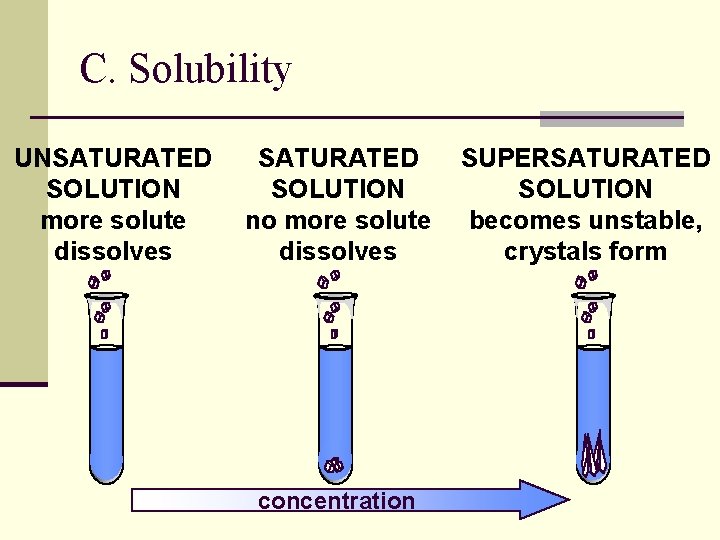
Saturation of Solutions Ø A solution that contains the maximum amount of solute that may be dissolved under existing conditions is saturated. Ø A solution that contains less solute than a saturated solution under existing conditions is unsaturated.

C. Solubility UNSATURATED SOLUTION more solute dissolves SATURATED SOLUTION no more solute dissolves concentration SUPERSATURATED SOLUTION becomes unstable, crystals form

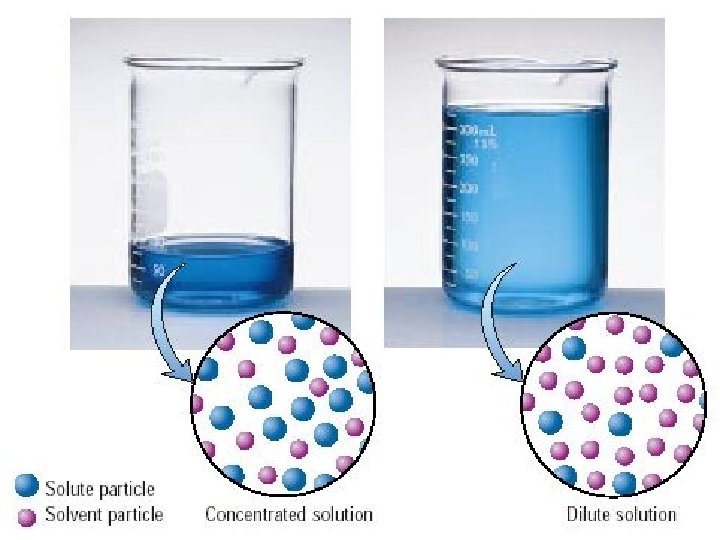
Concentrated vs. Dilute

Concentrated and Dilute • A concentrated solution contains more solute than solvent • A dilute solution contains more solvent than solute

Dissolution of sodium Chloride

Solubility Trends § The solubility of MOST solids increases with temperature. § The rate at which solids dissolve increases with increasing surface area of the solid. § The solubility of gases decreases with increases in temperature. § The solubility of gases increases with the pressure above the solution.

Therefore… Solids tend to dissolve best when: o Heated o Stirred o Ground into small particles Gases tend to dissolve best when: o The solution is cold o Pressure is high

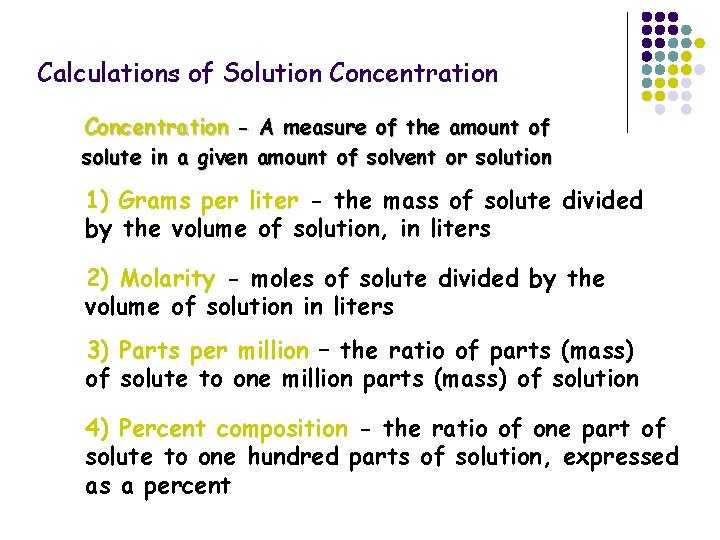
Calculations of Solution Concentration - A measure of the amount of solute in a given amount of solvent or solution 1) Grams per liter - the mass of solute divided by the volume of solution, in liters 2) Molarity - moles of solute divided by the volume of solution in liters 3) Parts per million – the ratio of parts (mass) of solute to one million parts (mass) of solution 4) Percent composition - the ratio of one part of solute to one hundred parts of solution, expressed as a percent

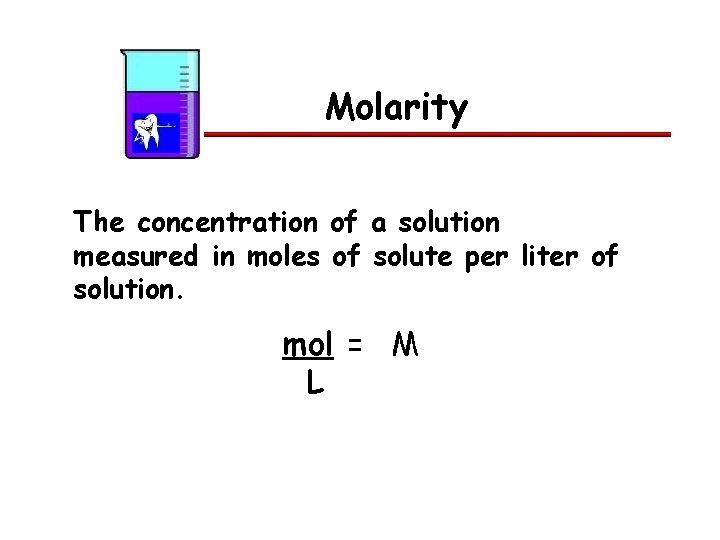
Molarity The concentration of a solution measured in moles of solute per liter of solution. mol = M L

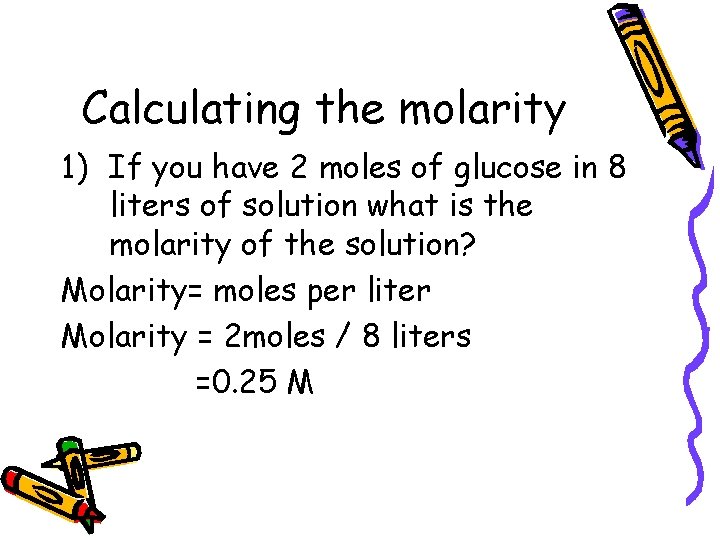
Calculating the molarity 1) If you have 2 moles of glucose in 8 liters of solution what is the molarity of the solution? Molarity= moles per liter Molarity = 2 moles / 8 liters =0. 25 M

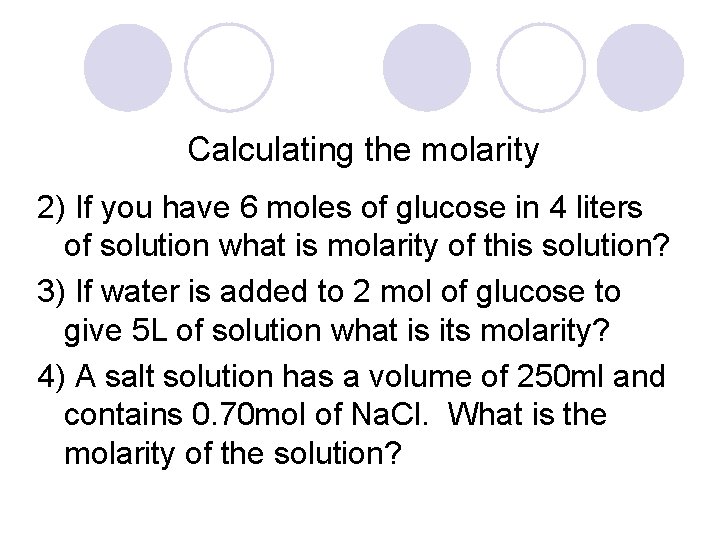
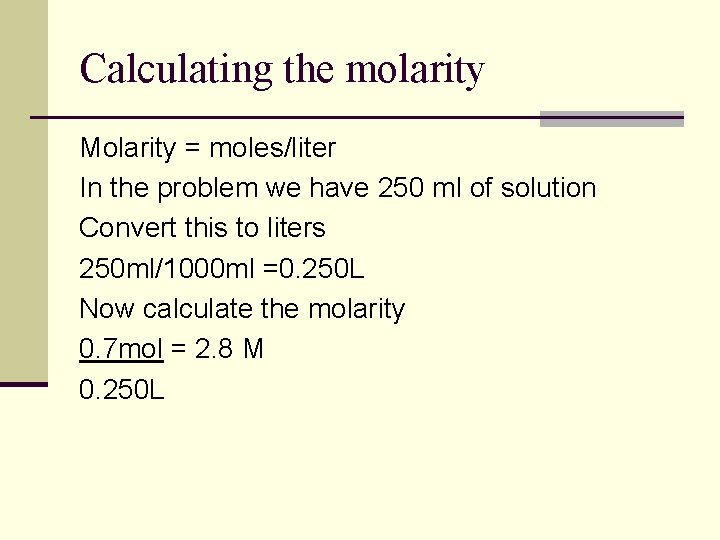
Calculating the molarity 2) If you have 6 moles of glucose in 4 liters of solution what is molarity of this solution? 3) If water is added to 2 mol of glucose to give 5 L of solution what is its molarity? 4) A salt solution has a volume of 250 ml and contains 0. 70 mol of Na. Cl. What is the molarity of the solution?

Calculating the molarity Molarity = moles/liter In the problem we have 250 ml of solution Convert this to liters 250 ml/1000 ml =0. 250 L Now calculate the molarity 0. 7 mol = 2. 8 M 0. 250 L

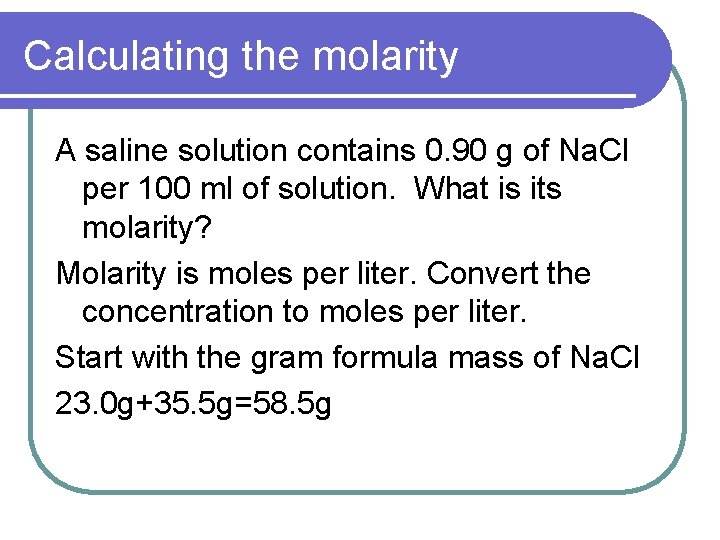
Calculating the molarity A saline solution contains 0. 90 g of Na. Cl per 100 ml of solution. What is its molarity? Molarity is moles per liter. Convert the concentration to moles per liter. Start with the gram formula mass of Na. Cl 23. 0 g+35. 5 g=58. 5 g

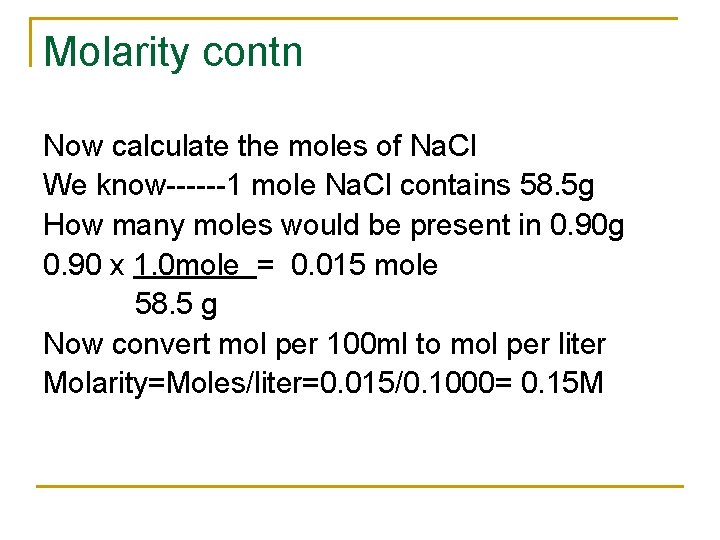
Molarity contn Now calculate the moles of Na. Cl We know------1 mole Na. Cl contains 58. 5 g How many moles would be present in 0. 90 g 0. 90 x 1. 0 mole = 0. 015 mole 58. 5 g Now convert mol per 100 ml to mol per liter Molarity=Moles/liter=0. 015/0. 1000= 0. 15 M

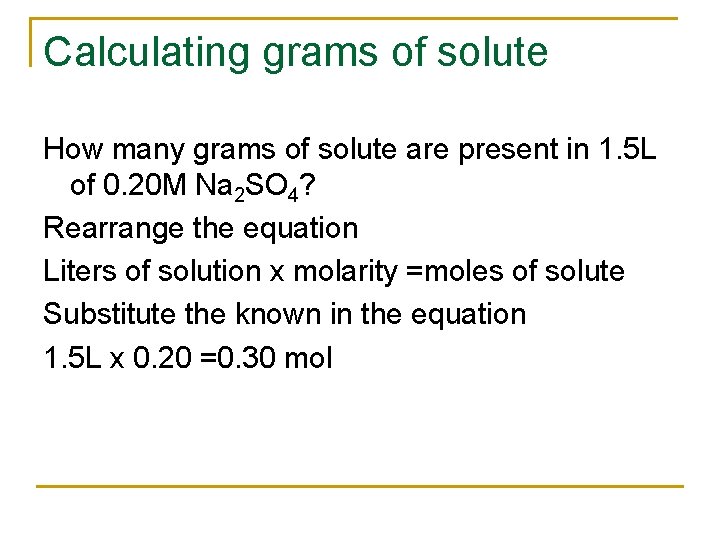
Calculating grams of solute How many grams of solute are present in 1. 5 L of 0. 20 M Na 2 SO 4? Rearrange the equation Liters of solution x molarity =moles of solute Substitute the known in the equation 1. 5 L x 0. 20 =0. 30 mol

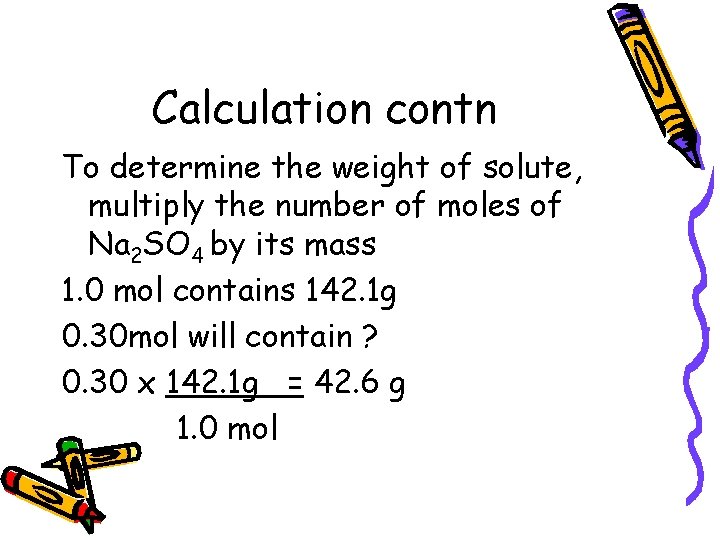
Calculation contn To determine the weight of solute, multiply the number of moles of Na 2 SO 4 by its mass 1. 0 mol contains 142. 1 g 0. 30 mol will contain ? 0. 30 x 142. 1 g = 42. 6 g 1. 0 mol
 Antigentest åre
Antigentest åre Are all solutions homogeneous mixtures
Are all solutions homogeneous mixtures Are all aqueous solutions homogeneous
Are all aqueous solutions homogeneous Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Solutions are homogeneous mixtures
Solutions are homogeneous mixtures Application of homogeneous differential equation
Application of homogeneous differential equation Types of mixtures
Types of mixtures Homogeneous mixtures examples
Homogeneous mixtures examples Common homogeneous mixtures
Common homogeneous mixtures Facts about homogeneous mixtures
Facts about homogeneous mixtures Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Section 1 composition of matter
Section 1 composition of matter What is mechanical mixtures
What is mechanical mixtures Types of matter elements compounds and mixtures
Types of matter elements compounds and mixtures How can matter be classified
How can matter be classified Are solutions homogeneous
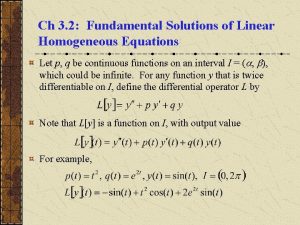
Are solutions homogeneous Fundamental solutions of linear homogeneous equations
Fundamental solutions of linear homogeneous equations Are solutions homogeneous
Are solutions homogeneous Homogeneous mixture
Homogeneous mixture Fundamental solutions of linear homogeneous equations
Fundamental solutions of linear homogeneous equations Is salad a mixture or solution
Is salad a mixture or solution Chapter 14 mixtures and solutions
Chapter 14 mixtures and solutions Chapter 13 solutions chemistry
Chapter 13 solutions chemistry Chapter 14 mixtures and solutions worksheet answers
Chapter 14 mixtures and solutions worksheet answers