Solutions Classification of Matter MATTER Mixtures a physical






- Slides: 20

Solutions!

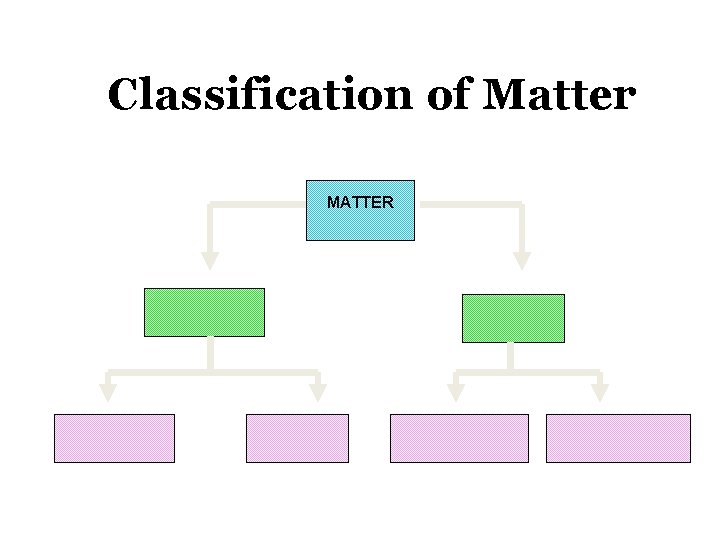
Classification of Matter MATTER

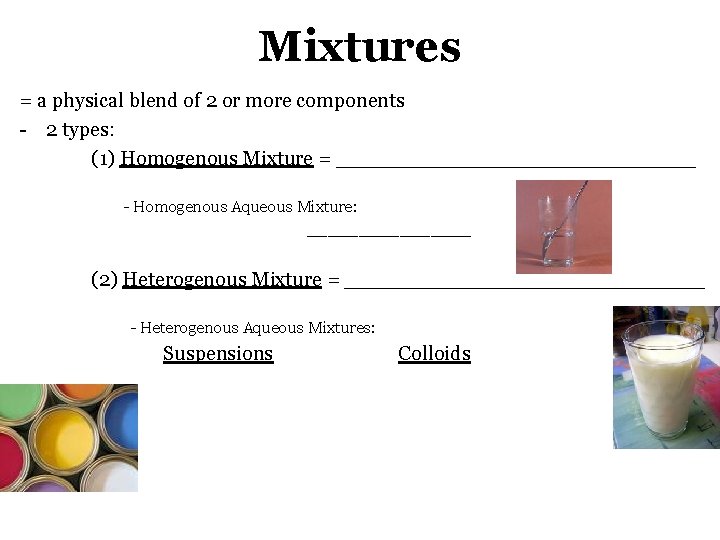
Mixtures = a physical blend of 2 or more components - 2 types: (1) Homogenous Mixture = ______________ - Homogenous Aqueous Mixture: ________ (2) Heterogenous Mixture = ______________ - Heterogenous Aqueous Mixtures: Suspensions Colloids

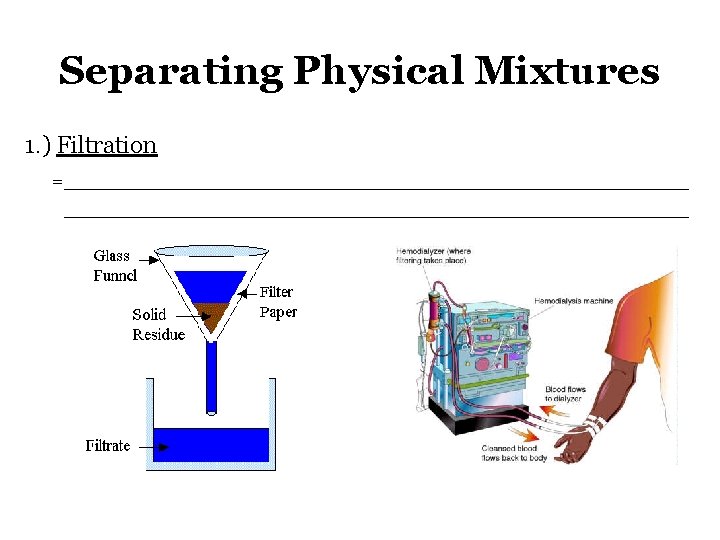
Separating Physical Mixtures 1. ) Filtration =______________________________________________________


Solutions = a homogenous mixture of substances in the same physical state **We will be discussing aqueous solutions mostly. ** __________ Solvent =____________ Solute = ____________ can be atoms, ions, or molecules

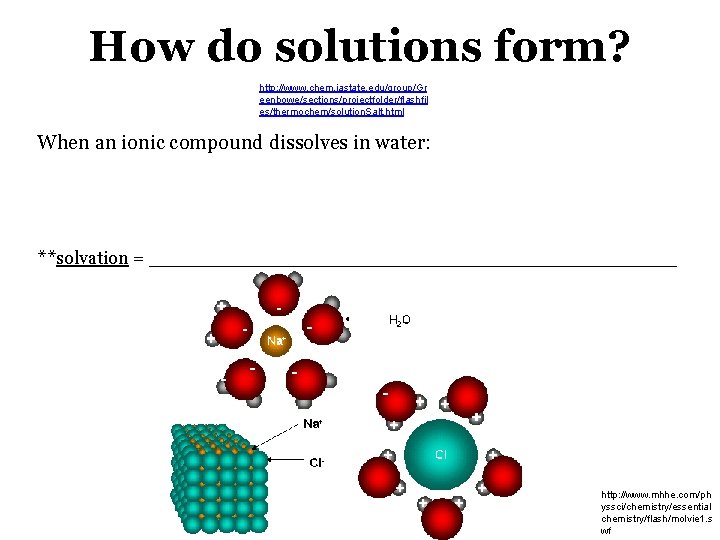
How do solutions form? http: //www. chem. iastate. edu/group/Gr eenbowe/sections/projectfolder/flashfil es/thermochem/solution. Salt. html When an ionic compound dissolves in water: **solvation = _____________________ http: //www. mhhe. com/ph yssci/chemistry/essential chemistry/flash/molvie 1. s wf

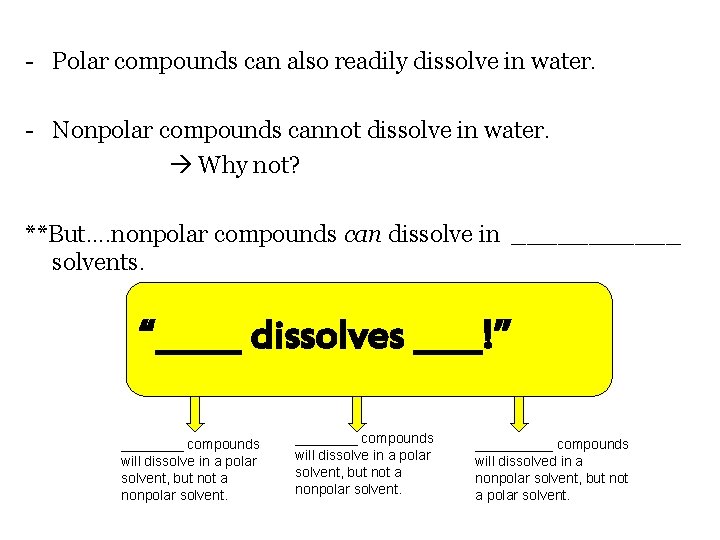
- Polar compounds can also readily dissolve in water. - Nonpolar compounds cannot dissolve in water. Why not? **But…. nonpolar compounds can dissolve in ______ solvents. “_____ dissolves ____!” ________ compounds will dissolve in a polar solvent, but not a nonpolar solvent. _____ compounds will dissolved in a nonpolar solvent, but not a polar solvent.

- Electrolyte = _________________________________________ **All ionic compounds are electrolytes because: ____________________ - Nonelectrolyte = _________________________________________ **Most compounds are nonelectrolytes because: _____________________

Factors Affecting How Fast Particles Dissolve 1. ) Stirring makes compounds dissolve faster. 2. ) Higher temperatures make compounds dissolve faster. 3. ) Smaller-sized particles dissolve faster.

Solubility - Solubility = ___________________________________________ - usually expressed in grams of solute per 100 g of solvent - Factors Affecting Solubility 1. ) Nature of solute & solvent: **Remember: “Like dissolves like. ”**

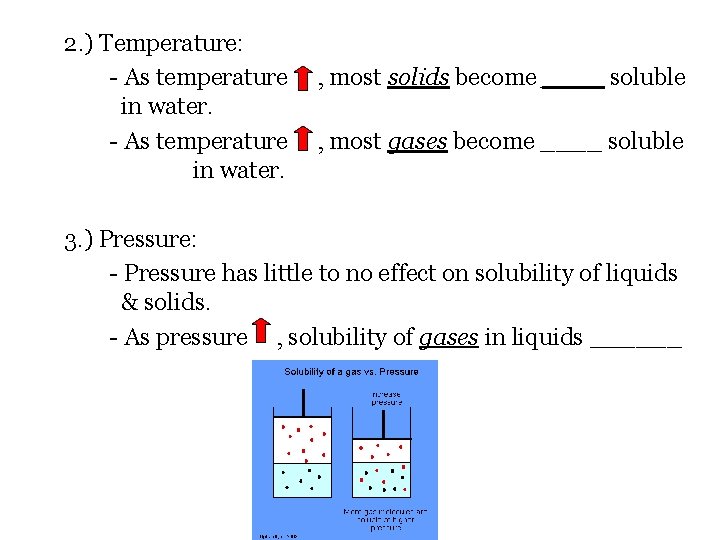
2. ) Temperature: - As temperature in water. , most solids become ____ soluble , most gases become ____ soluble 3. ) Pressure: - Pressure has little to no effect on solubility of liquids & solids. - As pressure , solubility of gases in liquids ______

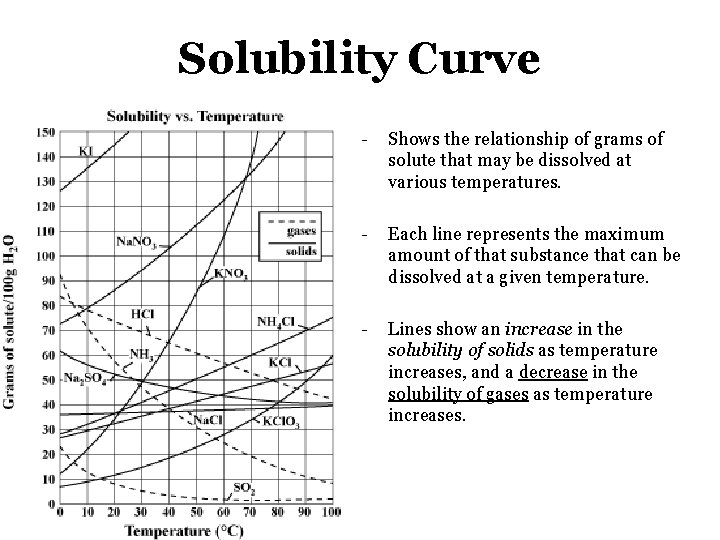
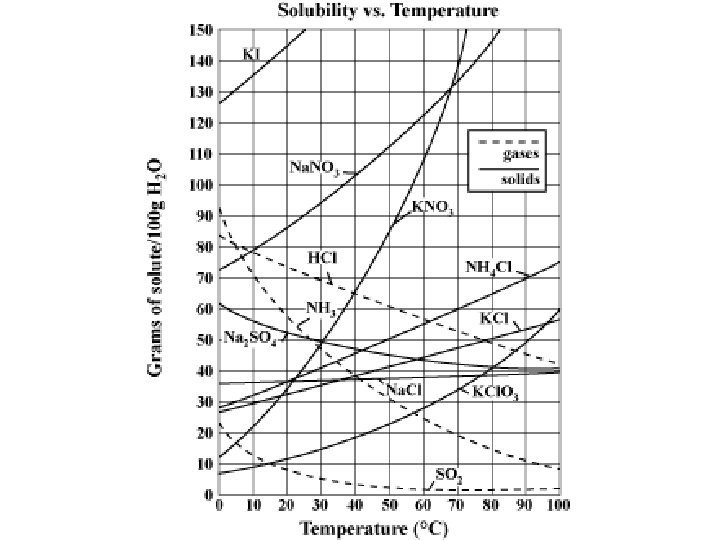
Solubility Curve - Shows the relationship of grams of solute that may be dissolved at various temperatures. - Each line represents the maximum amount of that substance that can be dissolved at a given temperature. - Lines show an increase in the solubility of solids as temperature increases, and a decrease in the solubility of gases as temperature increases.

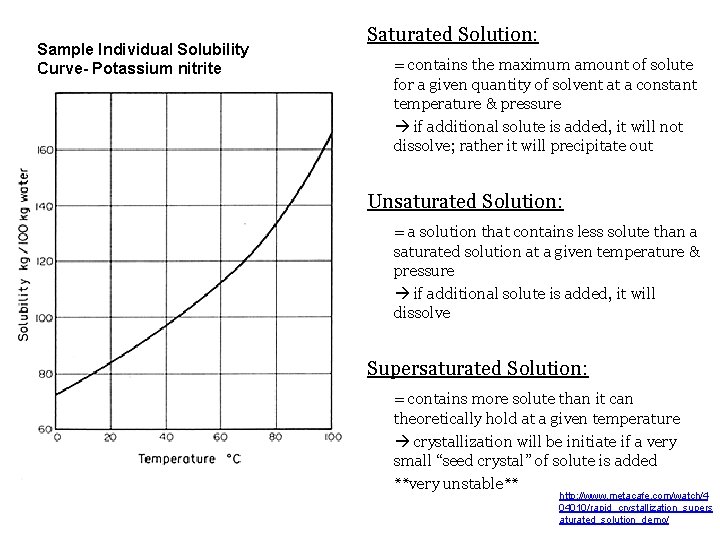
Sample Individual Solubility Curve- Potassium nitrite Saturated Solution: = contains the maximum amount of solute for a given quantity of solvent at a constant temperature & pressure if additional solute is added, it will not dissolve; rather it will precipitate out Unsaturated Solution: = a solution that contains less solute than a saturated solution at a given temperature & pressure if additional solute is added, it will dissolve Supersaturated Solution: = contains more solute than it can theoretically hold at a given temperature crystallization will be initiate if a very small “seed crystal” of solute is added **very unstable** http: //www. metacafe. com/watch/4 04010/rapid_crystallization_supers aturated_solution_demo/


Colligative Properties of Solutions Colligative Property = ________________________ Vapor Pressure Lowering Boiling Point Elevation Freezing Point Depression

Vapor Pressure Lowering Remember: Vapor pressure = _________________ **A solution always has a lower vapor pressure than the pure solvent. ** Why?

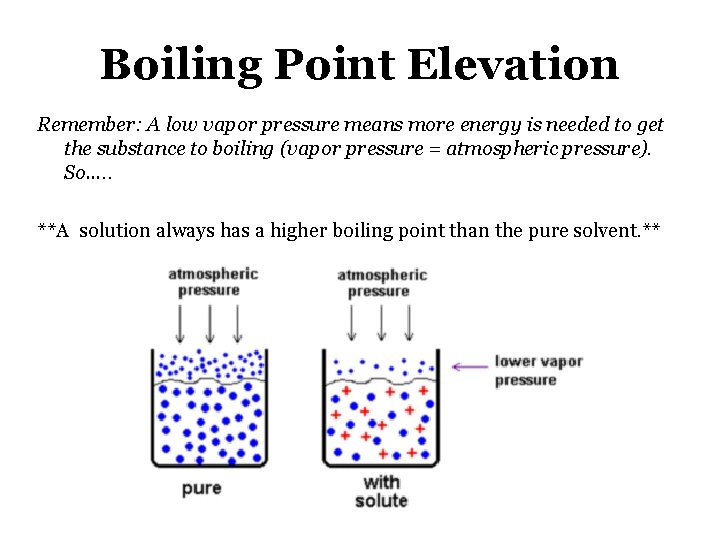
Boiling Point Elevation Remember: A low vapor pressure means more energy is needed to get the substance to boiling (vapor pressure = atmospheric pressure). So…. . **A solution always has a higher boiling point than the pure solvent. **

Freezing Point Depression **A solution always has a lower freezing point than the pure solvent. ** Why? ? ? Vs.

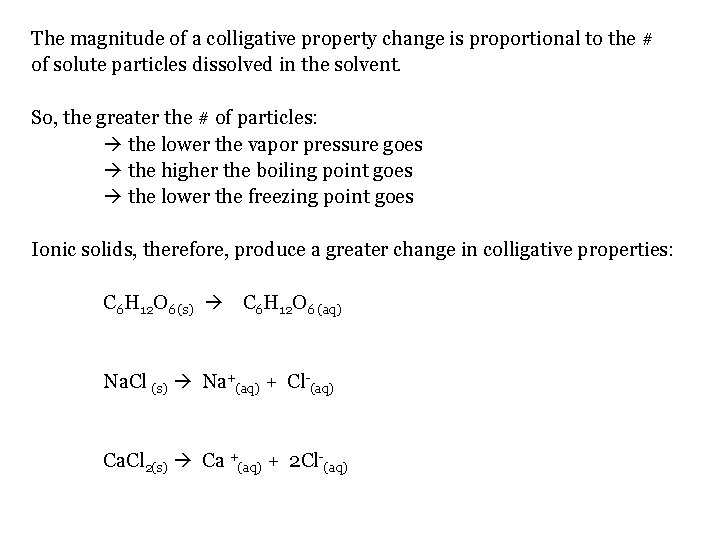
The magnitude of a colligative property change is proportional to the # of solute particles dissolved in the solvent. So, the greater the # of particles: the lower the vapor pressure goes the higher the boiling point goes the lower the freezing point goes Ionic solids, therefore, produce a greater change in colligative properties: C 6 H 12 O 6 (s) C 6 H 12 O 6 (aq) Na. Cl (s) Na+(aq) + Cl-(aq) Ca. Cl 2(s) Ca +(aq) + 2 Cl-(aq)
 Are all solutions homogeneous mixtures
Are all solutions homogeneous mixtures Are all solutions mixtures
Are all solutions mixtures Chapter 14 mixtures and solutions
Chapter 14 mixtures and solutions Chapter 13 solutions chemistry
Chapter 13 solutions chemistry Colloids mixture
Colloids mixture Mixtures solubility and acid/base solutions answer key
Mixtures solubility and acid/base solutions answer key How many elements
How many elements Fossweb mixtures and solutions
Fossweb mixtures and solutions Pure substance examples
Pure substance examples Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Are all aqueous solutions homogeneous
Are all aqueous solutions homogeneous Mixtures and solutions quiz
Mixtures and solutions quiz Solutions are homogeneous mixtures
Solutions are homogeneous mixtures Ns grade 6 term 2
Ns grade 6 term 2 Solutions are heterogenous mixtures
Solutions are heterogenous mixtures Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Mechanical mixtures
Mechanical mixtures Classifying elements compounds and mixtures
Classifying elements compounds and mixtures Matter is classified as ____ and mixtures
Matter is classified as ____ and mixtures







































