SOLIDLIQUID INTERACTIONS Zeroorder reactions have a constant rate























- Slides: 38

SOLID-LIQUID INTERACTIONS

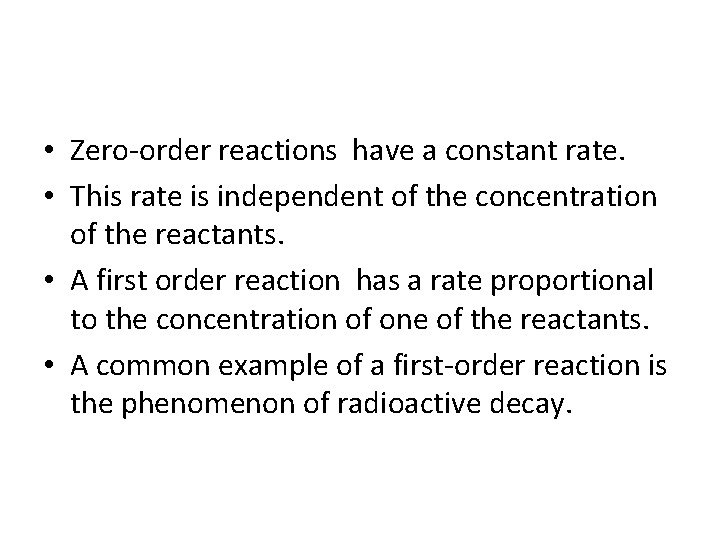
• Zero-order reactions have a constant rate. • This rate is independent of the concentration of the reactants. • A first order reaction has a rate proportional to the concentration of one of the reactants. • A common example of a first-order reaction is the phenomenon of radioactive decay.

Zero-order reactions • A 0 -order reaction has a rate which is independent of the concentration of the reactant(s). • Increasing the concentration of the reacting species will not speed up the rate of the reaction. • In zero-order reactions a material that is required for the reaction to proceed, • Such as a surface or a catalyst, is saturated by the reactants • A reaction is zero order if concentration data are plotted versus time and the result is a straight line

First-order reactions • A first-order reaction depends on the concentration of only one reactant • (a unimolecular reaction). Other reactants can be present, but each will be zero-order.

• Solidification and melting are transformations between crystallographic and noncrystallographic states of a metal or alloy. • These transformations are of course basic to such technological applications as • Ingot casting, foundry casting, continuous casting, single-crystal growth for semiconductors, • More recently directionally solidified composite alloys

• Another important and complex solidification and melting process, often neglected concerns the process of fusion welding. • An understanding of the mechanism of solidification and how it is affected by • Such parameters as temperature distribution, cooling rate and alloying, • Is important in the control of mechanical properties of cast metals and fusion welds.

• The objective our lecture is to develop some of the basic concepts of solidification, • Apply these to the practical processes of ingot casting, continuous casting and fusion welding. • We then consider a few practical examples illustrating the casting of engineering alloys in the light of theoreticalbackground.

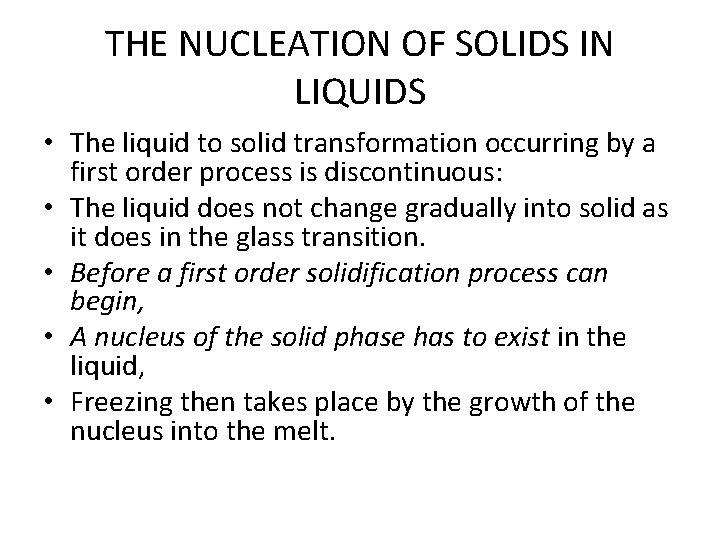
THE NUCLEATION OF SOLIDS IN LIQUIDS • The liquid to solid transformation occurring by a first order process is discontinuous: • The liquid does not change gradually into solid as it does in the glass transition. • Before a first order solidification process can begin, • A nucleus of the solid phase has to exist in the liquid, • Freezing then takes place by the growth of the nucleus into the melt.

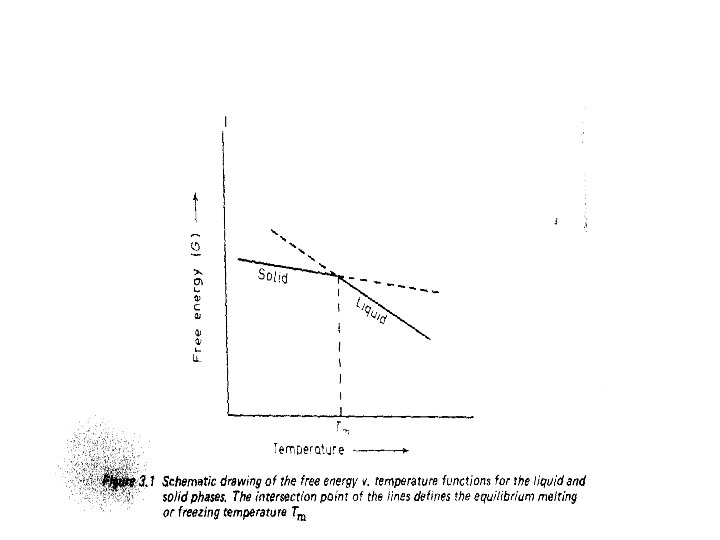
• Solidification in this sense requires the simultaneous presence of both the solid and the liquid phases • An important feature of crystalline solids is that • At constant pressure they have a characteristic equilibrium freezing/melting temperature, Tm.


• The solid and the liquid phases can only be in thermodynamic equilibrium at this unique temperature. • we can also say that at the equilibrium freezing temperature • The free energies of the solid and liquid phases are equal. • At temperatures above Tm , the liquid has a lower free energy than the solid, • And the liquid is therefore the stable phase. • At temperatures below Tm the solid has the lower free energy and is therefore the stable phase.

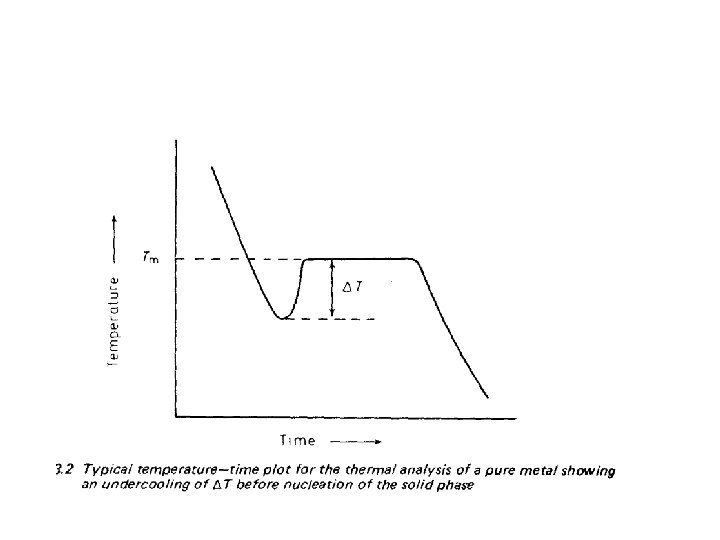
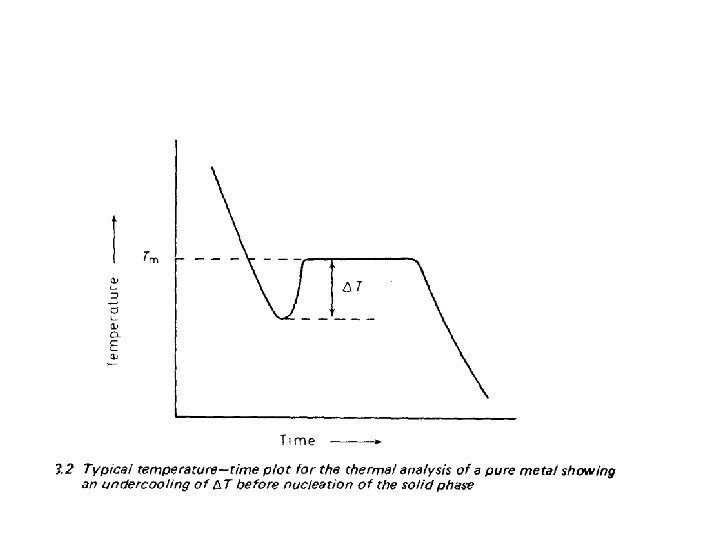
• when a liquid reached to a temperature below its thermodynamic freezing temperature • It not immediately and spontaneously crystallize • A certain degree of “undercooling’ or ‘super cooling’ of the liquid is usually necessary before the freezing process starts. • If the undercooling is actually measured, a time— temperature graph such as is shown in Figure 3. 2 is obtained.



• The liquid first under cools an amount T, • At this temperature the solid nucleates: • Growth then proceeds with the evolution of latent heat, • The temperature rises to equilibrium freezing temperature • ( more accurately, to slightly below equilibrium freezing temperature,

• Since a small under cooling is required to drive the process in the direction of solidification. • When solidification is complete and no more latent heat is evolved, • The temperature of the solid continues to fall. • From this description of the freezing process, two questions immediately arises.

• (1) what are crystal nuclei? and • (2) why do they appear only at temperatures significantly less than the equilibrium freezing temperature? • To answer these questions we again have to consider the • i) Atomic structure of liquids and solids, • Ii) Thermodynamic energy requirements for the formation of a small crystal of the solid phase in the bulk liquid phase.


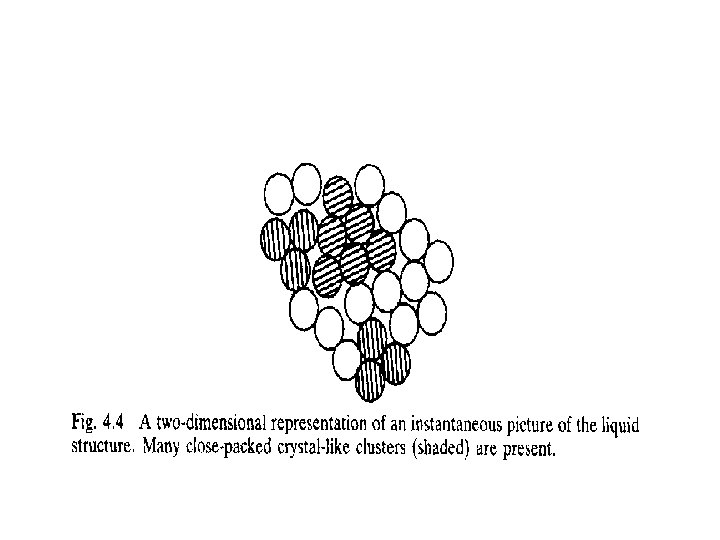
Homogeneous Nucleation • Consider a certain volume of liquid being progressively cooled. • The atoms of the liquid are in a state of constant agitation, • Therefore, the liquid exhibits no permanent long range order, • It is probable that some atoms will cluster together momentarily into small regions having the crystal structure of the solid phase (embryos). • These small clusters, called embryos, are potential nuclei for the solidification process. • We can determine if the embryos will become actual nuclei by considering the free energy changes involved in their formation.


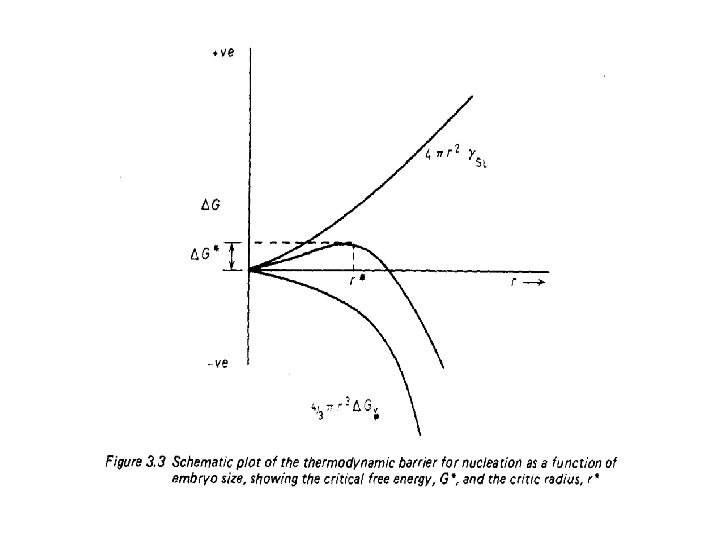
• If GL and Gs are the free-energies per unit volume of the liquid and solid phases respectively, • the difference between GL and Gs ( Delta Gv) provides a negative energy contribution at temperatures less than TE (see Figure 3. 1). • There is, however, a positive energy term due to the creation of the solid—liquid interface. • Let gamma. SL represent the (positive) energy per unit area of the surface dividing the solid embryo from the liquid. • The change in free energy corresponding to the formation of a sphere of solid of radius r is then given by

• The important thing to notice about this equation is that the surface energy term varies as the square of the radius, • whereas the volume free energy term varies as the cube. • The change in free energy varies with the size of solid sphere in a manner shown in Figure 3. 3.


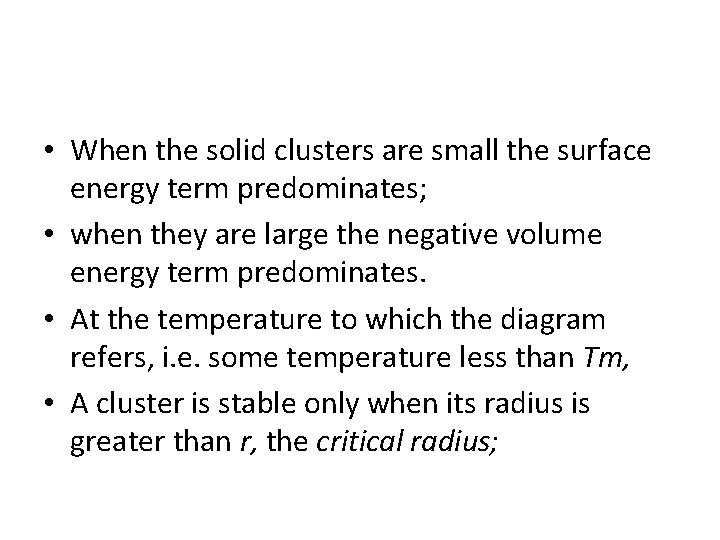
• When the solid clusters are small the surface energy term predominates; • when they are large the negative volume energy term predominates. • At the temperature to which the diagram refers, i. e. some temperature less than Tm, • A cluster is stable only when its radius is greater than r, the critical radius;

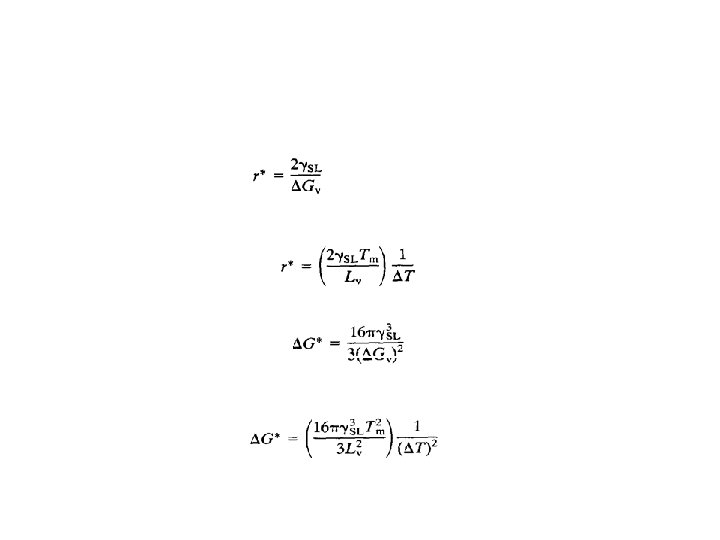
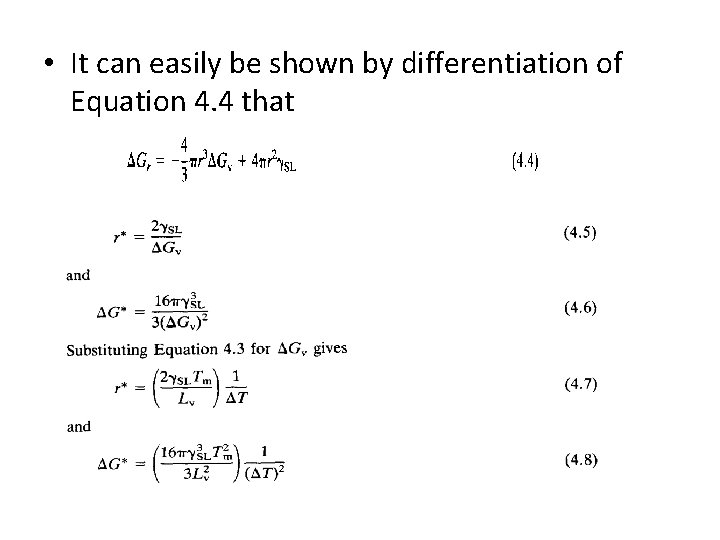
• The free energy of sub-critical clusters would have to increase if they were to grow, • This is thermodynamically impossible. • Consequently, embryos of radius less than r* disappear, • Nuclei of radius greater than r grow larger. • The critical radius can be found from the equation 4. 4 • By differentiating G with respect to r and setting the derivative equal to zero.


• It can easily be shown by differentiation of Equation 4. 4 that

• The above formulation tells us what size embryo is required to form a critical nucleus at any temperature, • But it does not say whether embryos of that size actually exist in the liquid at that temperature. • Intuitively, we would expect a range of embryo sizes in the liquid at any temperature, and

• We would further anticipate that at the higher the temperature the smaller would be the largest -size embryos • Because of the increased amplitude of atomic vibrations at the higher temperatures. • It is also reasonable to assume that the larger the volume of liquid, the greater is the probability of an embryo of critcal size existing.

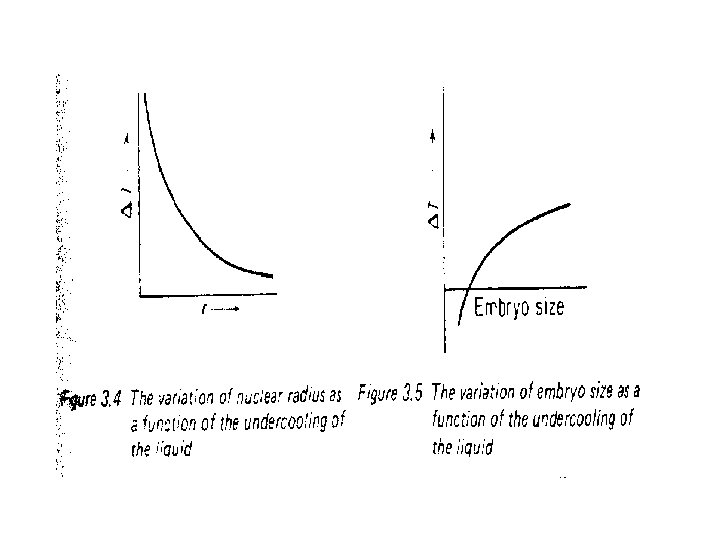
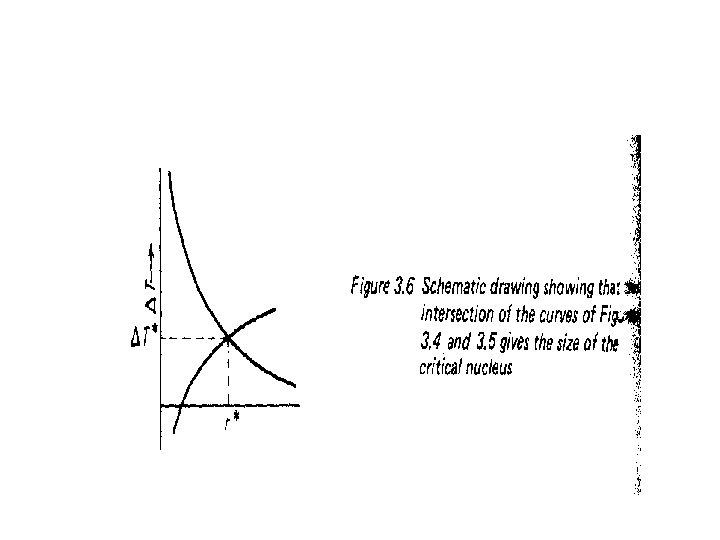
• Figure 3. 4 the larger the supper cooling the smaller the critical nucleus size. • Figure 3. 5 shows the expected form of the ‘relationship between the maximum embryo size and temperature, • A relationship we could confirm if we were to follow the argument through mathematically • By superimposing Figure 3. 5 onto Figure 3. 4, • The point of intersection of the curves represents the temperature at which embryos become viable crystal nuclei.


• Figure 3. 6 is, therefore, the temperature at which solid will nucleate spontaneously in the liquid, • And is called the homogeneous nucleation temperature. • It has been found empirically that for simple liquids, such as liquid metals, • The amount of undercooling required for homogeneous nucleation • Is an approximately constant fraction of the melting point (expressed in K ) • For each material, T – O. 2 O Tm. The basic reason for this large barrier t:


• The basic reason for this large barrier nucleation is the great difference in structure between the solid and liquid phases, • The solid consisting of a regular arrangement of atoms on a crystal lattice • whereas the liquid is best described as amorphous. • Delta. T(critical) is obviously the maximum undercooling attainable • Since by definition a liquid cannot be (slowly) supercooled to a temperature less than T*

• For the high temperature metals Delta T amounts to several hundred degrees centigrade: • The homogeneous nucleation temperature of pure iron, for instance, is approximately 300 K below its equilibrium freezing temperature. • From Equation (3. 3) it can be calculated that the critical size nucleus for homogeneous nucleation contains in the region of 300 atoms

• It is known that the presence of foreign solids in the liquid, or • Even the walls of the container in which the liquid is held, • may serve to nucleate the solid heterogeneously at a temperature considerably above the homogeneous nucleation temperature. • Using the example of commercial steels cast into ingot moulds,

• we know from experience that • The solid nucleates in the vicinity of the mould walls at a temperature of only a few degrees less than the equilibrium freezing temperature • The mould walls serve to chill the liquid locally and • Thus permit small solid particles in suspension in the liquid to act as heterogeneous nucleation centres for the freezing process. • Solidification begins at the outside of the ingot and progresses inwards with time.

• The purest commercial metals and alloys always contain sufficient foreign bodies, or inclusions, • To serve as heterogeneous nucleation catalysts even if the mould walls are themselves inert in this respect. • Very rarely, and then only under the most careful experimental conditions, will a liquid supercool to its homogeneous nucleation temperature.